A case report on phenobarbitone induced stevens-johnson syndrome: an alarming hypersensitivity reaction
Abstract
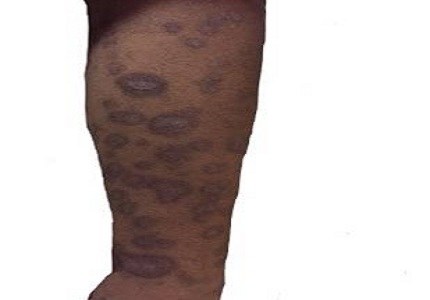
Stevens-Johnson syndrome (SJS) is an Ig E mediated hypersensitivity reaction, sometimes complicated by ocular manifestations. Antiepileptics induced SJS is common with carbamazepine. Phenobarbitone is known to cause hypersensitivity reactions like mild to moderate rashes but not life-threatening reactions like SJS. We present a case report of an 18 years old female patient who presented with chief complaints of multiple, fluid-filled lesions associated with itching all over the body for 20 days and inability to open her eyes for 18 days. It had developed following ingestion of Tab. Phenobarbitone 30 mg orally BD for 15 days. She was diagnosed as a case of SJS and treated with parenteral followed by oral corticosteroids and antihistaminics and recovered over a span of 20 days. Causality was assessed as per WHO-UMC Causality Assessment criteria and Naranjo Scale. Main pathogenesis is apoptosis through an interaction between cell-surface death receptor like Fas and its receptive ligand or due to genetic deficiency. In case of aromatic anticonvulsants, cross reactivity is also suspected. Genetic studies and cross reactivity testing can help prevent further incidences in few.
Downloads
References
Gaur S, Agnihotri R. Phenobarbital induced Stevens–Johnson syndrome in a child. Indian Journal of Pharmacology. 2012;44(4):531-532.
Deore SS, Dandekar RC, Mahajan AM, Shiledar VV. Drug Induced - Stevens Johnson Syndrome: A Case Report. Int J Sci Stud. 2014;2(4):84-87.
Ramineni HB, Eluri P, Vipparla K, Suryadevara V. Phenobarbital induced Stevens Johnson syndrome: a case report. Int J Res Med Sci. 2015;3:492-3.
Sean C Sweet man. Martindale: The Complete Drug Reference. 36th ed. London:Pharmaceutical Press; 2009.Antiepileptics:492-495.
Stevens–Johnson syndrome. (2017, October 25). In Wikipedia, The Free Encyclopedia. Retrieved 07:10, October 30, 2017, from https://en.wikipedia.org/w/index.php?title=Stevens%E2%80%93Johnson_syndrome&oldid=807068005.
Mockenhaupt M, Messenheimer J, Tennis P, Schlingmann J. Risk ofStevens-Johnson syndrome and toxic epidermal necrolysis in newusers of antiepileptics. Neurology.2005Apr12;64(7):1134-8.
Trivedi BS, Darji NH, Malhotra SD, Patel PR. Antiepileptic Drugs-induced Stevens–Johnson syndrome: A case Series. Journal of Basic and Clinical Pharmacy. 2016;8(1):42-44.
WHO. The use of the WHO-UMC system for standardized case causality assessment, 2014. Available at: http://www.WHOUMC.org/graphics/4409.pdf
Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, Janecek E, Domecq C, Greenblatt DJ. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981 Aug;30(2):239-45.
Lee Y-J, Yum M-S, Kim E-H, Ko T-S. Intravenous levetiracetam versus phenobarbital in children with status epilepticus or acute repetitive seizures. Korean Journal of Pediatrics. 2016;59(1):35-39.


 OAI - Open Archives Initiative
OAI - Open Archives Initiative


