A comparative study of Olopatadine and Ketorolac eye drop with Ketorolac eye drop alone in seasonal allergic conjunctivitis
Abstract
Background: Seasonal allergic conjunctivitis is the most common allergic disorder seen in eyes. The aim of study was to compare the clinical efficacy of combination of 0.4% ketorolac and 0.1% olopatadine with 0.4% ketorolac alone in seasonal allergic conjunctivitis.
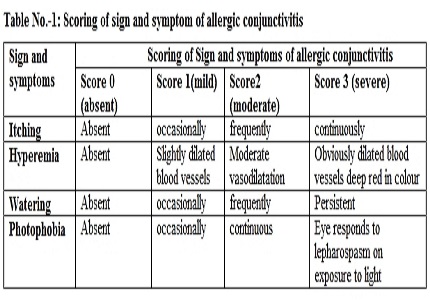
Material and Method: The study was prospective, double blind parallel group comparative. Two hundred cases enrolled in the study. All the subjects were randomly divided in two groups, 100 in each. Group 1 patients received 0.4% ketorolac eye drop in both eyes 2 times a day and group 2 patients received combination of 0.1% olopatadine and 0.4% ketorolac in both eyes 2 times a day. Observations were collected at baseline and on day 3,7,15 and analyzed statistically regarding improvement in sign and symptoms.
Result: In group 1, 50- 60% patients had no sign and symptoms on day 15 whereas in group 2 more than 95% patients showed improvement in clinical picture. p value was significant (p<0.0001) at day 15 in all sign and symptoms and on day 3 in itching and on day 7 in watering. Overall group 2 patients had better and earlier response regarding symptoms of itching at day 3.
Conclusion: The combination of 0.1% olopatadine and 0.4% ketorolac was more effective than 0.4% ketorolac alone in seasonal allergic conjunctivitis patients.
Downloads
References
Castillo M, Scott NW, Mustafa MZ, Mustafa MS, Azuara-Blanco A. Topical antihistamines and mast cell stabilisers for treating seasonal and perennial allergic conjunctivitis. Cochrane Database Syst Rev. 2015 Jun 1;(6):CD009566. doi: https://doi.org/10.1002/14651858.CD009566.pub2.
Liu R, Wu X, Wang X, Gao J, Zhou J, Zhao Q. Efficacy of olopatadine hydrochloride 0.1%, emedastine difumarate 0.05%, and loteprednol etabonate 0.5% for Chinese children with seasonal allergic conjunctivitis: a randomized vehicle-controlled study. Int Forum Allergy Rhinol. 2017;7:393–398.
Uchio E. Treatment of allergic conjunctivitis with olopatadine hydrochloride eye drops. Clin Ophthalmol. 2008 Sep;2(3):525-31.
Yaylali V, Demirlenk I, Tatlipinar S, Ozbay D, Esme A, Yildirim C, Ozden S. Comparative study of 0.1% olopatadine hydrochloride and 0.5% ketorolac tromethamine in the treatment of seasonal allergic conjunctivitis. Acta Ophthalmol Scand. 2003 Aug;81(4):378-82.
Abelson MB, Schaefer K. Conjunctivitis of allergic origin: immunologic mechanisms and current approaches to therapy. Surv Ophthalmol. 1993 Jul-Aug;38 Suppl:115-32.
Mehmet Borazan,1 Aylin Karalezli,1 Yonca Aydin Akova etal. Efficacy of olopatadine HCI 0.1%, ketotifen fumarate 0.025%, epinastine HCI 0.05%,emedastine 0.05% and fluorometholone acetate 0.1% ophthalmic solutions for seasonal allergic conjunctivitis: a placebo controlled environmental trial. Acta Ophthalmol. 2009: 87: 549–554.
Bonini S. The early and late phases of the ocular allergic reaction. Presented at the Second International Symposium. Challenges, Strategies and Tools to Optimize the Management of Ocular Allergy 22–25 June 1999, Leeds Castle, Kent, UK.
Aguilar AJ. Comparative study of clinical efficacy and tolerance in seasonal allergic conjunctivitis management with 0.1% olopatadine hydrochloride versus 0.05% ketotifen fumarate. Acta Ophthalmol Scand Suppl. 2000;(230):52-5.
Bielory L. Update on ocular allergy treatment. Expert Opin Pharmacother. 2002 May;3(5):541-53.
Bonini S, Gramiccioni C, Bonini M, Bresciani M. Practical approach to diagnosis and treatment of ocular allergy: a 1-year systematic review. Curr Opin Allergy Clin Immunol. 2007 Oct;7(5):446-9.
Hong, J. et al. Ambient air pollution, weather changes, and outpatient visits for allergic conjunctivitis: A retrospective registry study. Sci. Rep. 6, 23858; doi: https://doi.org/10.1038/srep23858.
Berdy GJ, Abelson MB, George MA, Smith LM, Giovanoni RL. Allergic conjunctivitis: a survey of new antihistamines. J Ocul Pharmacol. 1991 Winter;7(4):313-24.
Leonardi A, Zafirakis P. Efficacy and comfort of olopatadine versus ketotifen ophthalmic solutions: a double-masked, environmental study of patient preference. Curr Med Res Opin. 2004 Aug;20(8):1167-73.
Irani AM, Butrus SI, Tabbara KF, Schwartz LB. Human conjunctival mast cells: distribution of MCT and MCTC in vernal conjunctivitis and giant papillary conjunctivitis. J Allergy Clin Immunol. 1990 Jul;86(1):34-40.
Verin P, Easty DL, Secchi A, Ciprandi G, Partouche P, Nemeth-Wasmer G, Brancato R, Harrisberg CJ, Estivin-Ebrardt C, Coster DJ, Apel AJ, Coroneo MT, Knorr M, Carmichael TR, Kent-Smith BT, Abrantes P, Leonardi A, Cerqueti PM, Modorati G, Martinez M. Clinical evaluation of twice-daily emedastine 0.05% eye drops (Emadine eye drops) versus levocabastine 0.05% eye drops in patients with allergic conjunctivitis. Am J Ophthalmol. 2001 Jun;131(6):691-8.
Torkildsen G. Effective ocular allergy treatments are dual action. Ophthalmology Times, 1 April (2006).
Avunduk AM, Tekelioglu Y, Turk A, Akyol N. Comparison of the effects of ketotifen fumarate 0.025% and olopatadine HCl 0.1% ophthalmic solutions in seasonal allergic conjunctivitis:a 30‑day, randomized, double-masked artificial tear substitute-controlled trial. Clin Ther. 27(9), 1392–1402 (2005).
Kurt RA, Ucakhan-Gunduz M, Gunduz K.Olopatadine 0.1% and 0.2% ophthalmic solution for the management of ocular allergy Expert Rev. Opthamol.2010; 5(3), 287–296.
Abelson MB & Spitalny L. Combined analysis of two studies using the conjunctival allergen challenge model to evaluate olopatadine,a new ophthalmic antiallergic agent with dual activity. AmJ Ophthalmol 1998, 125: 797–804.
Deschenes J, Discepola M, Abelson M. Comparative evaluation of olopatadine ophthalmic solution (0.1%) versus ketorolac ophthalmic solution (0.5%) using the provocative antigen challenge model. Acta Ophthalmol Scand Suppl. 1999;(228):47-52.
Sharif NA, Xu SX, Miller ST, Gamache DA &Yanni JM. Characterization of theocular antiallergic and antihistaminic effects of olopatadine (AL-4943A), a novel drug for treating ocular allergic diseases. J Pharmacol Exp Ther 1996, 278: 1252–1261.
Yanni JM, Stephens DJ, Miller ST, Weimer LK, Graff G, Parnell D, Lang LS, Spellman JM, Brady MT, Gamache DA. The in vitro and in vivo ocular pharmacology of olopatadine (AL-4943A), an effective anti-allergic/antihistaminic agent. J Ocul Pharmacol Ther. 1996 Winter;12(4):389-400.
Wong AH, Barg SS, Leung AK. Seasonal and perennial allergic conjunctivitis. Recent Pat Inflamm Allergy Drug Discov. 2009 Jun;3(2):118-27.
Ostler HB: Vernal conjunctivitis. In Diseases of the external eye and adnexae: a text and atlas. 1st ed. Edited by Ostler HB. Baltimore: Williams & Wilkins; 1993:125.
Pavlos Michailopoulos,Dimitrios Gioulekas, Paschalina Giouleka etal Allergic conjunctivitis and the most common allergens in Northern GreeceWorld Aller Organ J. 2013; 6(1): 12 doi: https://doi.org/10.1186/1939-4551-6-12.
Leonardi A, De Dominicis C, Motterle L: Immunopathogenesis of ocular allergy: a schematic approach to different clinical entities. Curr Opin Allergy Clin Immunol 2007, 7(5):429–435.
Leonardi A: The central role of conjunctival mast cells in the pathogenesis of ocular allergy. Curr Allergy Asthma Rep 2002, 2(4):325–331.
Leonardi S, Marchese G, Marseglia GL, La Rosa M: Montelukast in allergic diseases beyond asthma. Allergy Asthma Proc 2007, 28(3):287–291. 37. Abelson MB, Paradis A, George MA, Smith LM, Maguire L, Burns R: Effects of.
Abelson MB, Paradis A, George MA, Smith LM, Maguire L, Burns R. Effects of Vasocon-A in the allergen challenge model of acute allergic conjunctivitis. Arch Ophthalmol. 1990 Apr;108(4):520-4.
Mishra GP, Tamboli V, Jawla J, Mitra AK: Recent patents and emerging therapeutics in the treatment of allergic conjunctivitis. Inflamm Allergy Drug Discov 2011, 5:26–36.
Kari O, Saari KM. Updates in the treatment of ocular allergies. J Asthma Allergy. 2010 Nov 24;3:149-58. doi: https://dx.doi.org/10.2147%2FJAA.S13705.
Spector SL, Raizman MB. Conjunctivitis medicamentosa. J Allergy Clin Immunol. 1994 Jul;94(1):134-6.
Dell SJ, Shulman DG, Lowry GM, Howes J: A controlled evaluation of the efficacy and safety of loteprednol etabonate in the prophylactic treatment of seasonal allergic conjunctivitis. Loteprednol Allergic Conjunctivitis Study Group. Am J Ophthalmol 1997, 123:791–797.
Dell SJ, Lowry GM, Northcutt JA, Howes J, Novack GD, Hart K: A randomized, double-masked, placebo-controlled parallel study of 0.2% loteprednol etabonate in patients with seasonal allergic conjunctivitis. J Allergy Clin Immunol 1998, 102:251–255.
Fan DS, Yu CB, Chiu TY, Wong CY, Ng JS, Pang CP, Lam DS. Ocular-hypertensive and anti-inflammatory response to rimexolone therapy in children. Arch Ophthalmol. 2003 Dec;121(12):1716-21.
Pflugfelder SC, Maskin SL, Anderson B, Chodosh J, Holland EJ, De Paiva CS,Bartels SP, Micuda T, Proskin HM, Vogel R: A randomized, double-masked,placebo-controlled, multicenter comparison of loteprednol etabonate ophthalmic suspension, 0.5%, and placebo for treatment of keratoconjunctivitis sicca in patients with delayed tear clearance. Am J Ophthalmol 2004, 138:444–457.
Comstock TL, Decory HH: Advances in corticosteroid therapy for ocular inflammation: loteprednol etabonate. Int J Inflam 2012. Epub 2012 Mar 28.
Maziak W, Behrens T, Brasky TM, Duhme H, Rzehak P, Weiland SK, Keil U: Are asthma and allergies in children and adolescents increasing. Results from ISAAC phase I and phase III surveys in Munster, Germany. Allergy 2003, 58:572–579.
Volkan Yaylali, Ibrahim Demirlenk, Sinan Tatlipinar etal Comparative study of 0.1% olopatadine hydrochloride and 0.5% ketorolac tromethamine in the treatment of seasonal allergic conjunctivitis Acta Ophthalmol. Scand. 2003: 81: 378–382.
Pallasaho P, Ronmark E, Haahtaela T, Sovijarvi ARA, Lundback B. Degree and clinical relevance of sensitization to common allergens among adults: a population study in Helsinki, Finland. Clin Exp Allergy. 2006;6:503–509. doi: https://doi.org/10.1111/j.1365-2222.2006.02460.x.
Raukas-Kivioja A, Raukas E, Loit HM, Kiviloogt J, Ronmmark E, Larssons K, Lundback B. Allergic sensitization among adults in Tallinn, Estonia. Clin Exp Allergy. 2003;6:1342–1348. doi: https://doi.org/10.1046/j.1365-2222.2003.01774.x.
Katelaris CH, Ciprandi G, Missotten L; International Olopatadine Study Group. 2002. A comparison of the effi cacy and tolerability of olopatadine hydrochloride 0.1% ophthalmic solution and cromolyn sodium 2% ophthalmic solution in seasonal allergic conjunctivitis. Clin Ther, 24:1561–75.
Tinkelman DG, Rupp G, Kaufman H, Pugely J, Schultz N. Double-masked, paired-comparison clinical study of ketorolac tromethamine 0.5% ophthalmic solution compared with placebo eyedrops in the treatment of seasonal allergic conjunctivitis. Surv Ophthalmol. 1993 Jul-Aug;38 Suppl:133-40.
Sarker Chowdhury, Hussain, Hossain & ChowdhuryComparison of the therapeutic efficacy of 0.1% olopatadine hydrochloride and 0.025% ketotifen fumarate in allergic conjunctivitisTherapy 2011; 8(5), 545–553.
Mortemousque B, Bourcier T, Khairallah M, Messaoud R, Brignole-Baudouin F, Renault D, Rebika H, Brémond-Gignac D; Ketotifen Study Group. Comparison of preservative-free ketotifen fumarate and preserved olopatadine hydrochloride eye drops in the treatment of moderate to severe seasonal allergic conjunctivitis. J Fr Ophtalmol. 2014 Jan;37(1):1-8. doi: https://doi.org/10.1016/j.jfo.2013.02.007. Epub 2013 Dec 31.
Donshik PC, Pearlman D, Pinnas J, Raizman MB, Tauber J, Tinkelman D, Walters TR..Efficacy and safety of ketorolac tromethamine 0.5% and levocabastine 0.05%: a multicenter comparison in patients with seasonal allergic conjunctivitis.Adv Ther. 2000 Mar-Apr;17(2):94-102.
Deschenes J, Discepola M, Abelson M. Comparative evaluation of olopatadine ophthalmic solution (0.1%) versus ketorolac ophthalmic solution (0.5%) using the provocative antigen challenge model.Acta Ophthalmol Scand Suppl. 1999;(228):47-5228.
Spangler DL, Bensch G, Berdy GJ. Evaluation of the efficacy of olopatadine hydrochloride 0.1% ophthalmic solution and azelastine hydrochloride 0.05% ophthalmic solution in the conjunctival allergen challenge model. Clin Ther. 2001 Aug;23(8):1272-80.
Lanier BQ, Finegold I, D'Arienzo P, Granet D, Epstein AB, Ledgerwood GL. Clinical efficacy of olopatadine vs epinastine ophthalmic solution in the conjunctival allergen challenge model. Curr Med Res Opin. 2004 Aug;20(8):1227-33.
McLaurin E, Narvekar A, Gomes P, Adewale A, Torkildsen G.Phase 3 Randomized Double-Masked Study of Efficacy and Safety of Once-Daily 0.77% Olopatadine Hydrochloride Ophthalmic Solution in Subjects With Allergic Conjunctivitis Using the Conjunctival Allergen Challenge Model.Cornea. 2015 Oct;34(10):1245-51. doi: https://doi.org/10.1097/ICO.0000000000000562.
Mah FS, Rosenwasser LJ, Townsend WD, Greiner JV, Bensch G. Efficacy and comfort of olopatadine 0.2% versus epinastine 0.05% ophthalmic solution for treating itching and redness induced by conjunctival allergen challenge. Curr Med Res Opin. 2007 Jun;23(6):1445-52. Epub 2007 May 18.


 OAI - Open Archives Initiative
OAI - Open Archives Initiative


