Comparison of survival outcome in early versus late surfactant therapy in preterm neonates with respiratory distress syndrome at a tertiary care centre: A randomized control trial (Open)
Abstract
Introduction: Prematurity and RDS largely contribute to early neonatal morbidity and mortality. With adequate antenatal steroid and early CPAP, early surfactant therapy improve survival outcome.
Material and Methods: Prospective interventional study included newborns with 24-28 weeks prematurity or 28-34 weeks(GA) with clinical RDS and birth weight(BW)>650gms. All subjects were preferably provided early surfactant therapy (within 2hours after birth). Surfactant (Curosurf) was delivered by INSURE technique (Intubate- Surfactant administration- Extubate) and only those who required further respiratory support were ventilated. Records on birth weight, gestational age, timing of therapy (early/late), duration of ventilation, sepsis, complications, and survival/death outcome were collected and data was analysed using SSPS version 17.
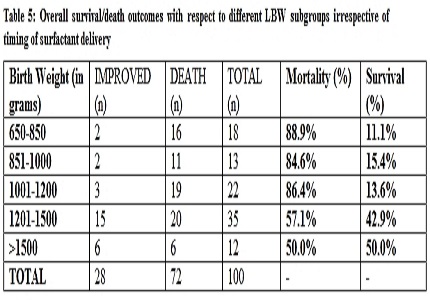
Results: Out of 100 neonates (49 male, 51 female), 46 received early surfactant therapy and 54 obtained it late; significantly more indoor patients could be treated early (p<0.0001). Although high mortality was observed with both early (65.2%) and late therapy (85.2%), there was significantly higher survival with early therapy (p=0.018). Though no statistical differences of outcome were observed with different GA and BW in study groups; irrespective of timing of therapy, higher mortality occurred in lower BW/GA subgroups with least survival among extremely preterms<27wks(p=0.000057) and ELBW<1000gm(p=0.013). No difference was seen for need of re-intubation/ventilation, but duration of ventilation was more on late group (p=0.043). Culture positive sepsis was found in 68% with higher association with late therapy (p=0.033). Hypotension was frequent complication with late intervention (p=0.029), whereas there was no difference for pulmonary hemorrhage or apnea.
Conclusion: Early surfactant administration improved survival with minimal complications in RDS except for extremely premature/LBW babies.
Downloads
References
Lawn JE, Cousens S, Zupan J, Lancet Neonatal Survival Steering Team. 4 million neonatal deaths: when? Where? Why? Lancet Lond Engl. 2005 Mar 5;365(9462):891–900.
Zupan, J, Ahman, E. Perinatal mortality for the year 2000: estimates developed by WHO. World Health Organization, Geneva; 2005.
Hack M, Fanaroff AA. Outcomes of extremely-low-birth-weight infants between 1982 and 1988. N Engl J Med. 1989 Dec 14;321(24):1642-7.
Hack M, Horbar JD, Malloy MH, Tyson JE, Wright E, Wright L. Very low birth weight outcomes of the National Institute of Child Health and Human Development Neonatal Network. Pediatrics. 1991 May;87(5):587–97.
Kumar P, Kumar R, Narang A. Spectrum of neonatal respiratory distress at PGI. Bull NNF. 1999;13:8–12.
Bhakoo ON. Assisted ventilation in neonates: the Indian perspective. Indian Pediatr. 1995 Dec;32(12):1261-4.
Nangia S, Saili A, Dutta AK, Gaur V, Singh M, Seth A, et al. Neonatal mechanical ventilation--experience at a level II care centre. Indian J Pediatr. 1998 Apr;65(2):291–6.
Engle WA, American Academy of Pediatrics Committee on Fetus and Newborn. Surfactant-replacement therapy for respiratory distress in the preterm and term neonate. Pediatrics. 2008 Feb;121(2):419–32.
Fujiwara T, Maeta H, Chida S, Morita T, Watabe Y, Abe T. Artificial surfactant therapy in hyaline-membrane disease. Lancet Lond Engl. 1980 Jan 12;1(8159):55–9.
Halliday HL. Surfactants: past, present and future. J Perinatol. 2008 May;28 Suppl 1:S47-56. doi: https://doi.org/10.1038/jp.2008.50.
Goldsmith LS, Greenspan JS, Rubenstein SD, Wolfson MR, Shaffer TH. Immediate improvement in lung volume after exogenous surfactant: alveolar recruitment versus increased distention. J Pediatr. 1991 Sep;119(3):424–8.
Alexander J, Milner AD. Lung volume and pulmonary blood flow measurements following exogenous surfactant. Eur J Pediatr. 1995 May;154(5):392-7.
Dani C, Ravasio R, Fioravanti L, Circelli M. Analysis of the cost-effectiveness of surfactant treatment (Curosurf®) in respiratory distress syndrome therapy in preterm infants: early treatment compared to late treatment. Ital J Pediatr. 2014 May 2;40:40.
Rebello CM, Precioso AR, Mascaretti RS, Grupo Colaborativo do Estudo Brasileiro Multicêntrico de Surfactante. A multicenter, randomized, double-blind trial of a new porcine surfactant in premature infants with respiratory distress syndrome. Einstein Sao Paulo Braz. 2014 Dec;12(4):397–404.
Gortner L, Wauer RR, Hammer H, Stock GJ, Heitmann F, Reiter HL, et al. Early versus late surfactant treatment in preterm infants of 27 to 32 weeks’ gestational age: a multicenter controlled clinical trial. Pediatrics. 1998 Nov;102(5):1153–60.
Verder H, Albertsen P, Ebbesen F, Greisen G, Robertson B, Bertelsen A, et al. Nasal continuous positive airway pressure and early surfactant therapy for respiratory distress syndrome in newborns of less than 30 weeks’ gestation. Pediatrics. 1999 Feb;103(2):E24.
Yost CC, Soll RF. Early versus delayed selective surfactant treatment for neonatal respiratory distress syndrome. Cochrane Database Syst Rev. 2000;(2):CD001456.
Ramanathan R. Surfactant therapy in preterm infants with respiratory distress syndrome and in near-term or term newborns with acute RDS. J Perinatol Off J Calif Perinat Assoc. 2006 May;26 Suppl 1:S51-56-64.
Velaphi, S. Early versus delayed selective surfactant treatment for neonatal respiratory distress syndrome: RHL commentary. [Internet]. World Health Organization, The WHO Reproductive Health Library, Geneva; 2010World Health Organization, The WHO Reproductive Health Library, Geneva; 2010; Available from: http://apps.who.int/rhl/newborn/cd001456_velaphis_com/en/. Accessed April 23, 2014.)
Bahadue FL, Soll R. Early versus delayed selective surfactant treatment for neonatal respiratory distress syndrome. Cochrane Database Syst Rev. 2012 Nov 14;11:CD001456.
Lopez E, Gascoin G, Flamant C, Merhi M, Tourneux P, Baud O; French Young Neonatologist Club. Exogenous surfactant therapy in 2013: what is next? Who, when and how should we treat newborn infants in the future? BMC Pediatr. 2013 Oct 10;13:165. doi: https://doi.org/10.1186/1471-2431-13-165.
Kandraju H, Murki S, Subramanian S, Gaddam P, Deorari A, Kumar P. Early routine versus late selective surfactant in preterm neonates with respiratory distress syndrome on nasal continuous positive airway pressure: a randomized controlled trial. Neonatology. 2013;103(2):148–54.
Jayachandra Naidu T,Kireeti AS,Lokesh B. Study of the outcome of early and late rescue surfactant administration in preterm babies. Asian J health sci. 2104 Dec;2(2):1–7.
Kim SM, Park YJ, Chung S-H, Choi Y-S, Kim CH, Bae C-W. Early prophylactic versus late selective use of surfactant for respiratory distress syndrome in very preterm infants: a collaborative study of 53 multi-center trials in Korea. J Korean Med Sci. 2014 Aug;29(8):1126–31.
Swarnkar K, Swarnkar M. Single dose surfactant early rescue therapy in respiratory distress syndrome-experience and outcome at a tertiary care centre. International Journal of Research in Medical Sciences(IJRMS). 2017 Jan;4(6):2107–11.
Reininger A, Khalak R, Kendig JW, Ryan RM, Stevens TP, Reubens L, et al. Surfactant administration by transient intubation in infants 29 to 35 weeks’ gestation with respiratory distress syndrome decreases the likelihood of later mechanical ventilation: a randomized controlled trial. J Perinatol Off J Calif Perinat Assoc. 2005 Nov;25(11):703–8.
Speer CP, Gefeller O, Groneck P, Laufkötter E, Roll C, Hanssler L, et al. Randomised clinical trial of two treatment regimens of natural surfactant preparations in neonatal respiratory distress syndrome. Arch Dis Child Fetal Neonatal Ed. 1995 Jan;72(1):F8-13.
M Garib, N Salama, S Deraz. Early versus late extubation after surfactant replacement therapy for respiratory distress syndrome. Egyptian Pediatric Association Gazette. 2015;63(1):1–5.
Bevilacqua G, Halliday H, Parmigiani S, Robertson B. Randomized multicentre trial of treatment with porcine natural surfactant for moderately severe neonatal respiratory distress syndrome. The Collaborative European Multicentre Study Group. J Perinat Med. 1993;21(5):329–40.


 OAI - Open Archives Initiative
OAI - Open Archives Initiative


