Evaluation of neutrophil elastase/ alpha-1-antitrypsin ratio in different stages of chronic obstructive pulmonary disease (COPD) patients
Abstract
Introduction: Little is known about protease, anti-protease markers in chronic obstructive pulmonary disease (COPD) patients.
Objectives: The objective of present study was to identify and try to correlate serum markers of protease and anti-protease with pulmonary functions in different stages of patients with COPD and to determine the ratio of neutrophil elastase/alpha-1-antitrypsin in different stages of COPD patients.
Methods: This prospective observational study was carried out in patients with stable COPD. Activities of serum alpha-1-antitrypsin and neutrophil elastase were measured in 220 stable COPD patients and in 60 healthy controls by ELISA method. 220 COPD patients were divided into 4 stages according to severity: stage I, II, III and IV.
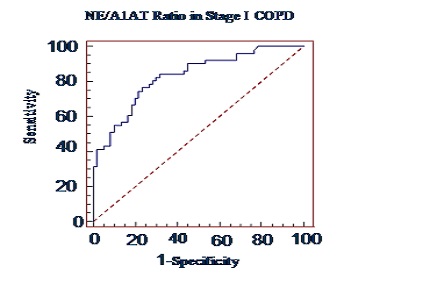
Results: An increase in serum neutrophil elastase and neutrophil elastase/alpha-1-antitrypsin ratio was observed in COPD patients with the advancement of the stage. In contrast to that, decreased activity of alpha-1-antitrypsin in serum was observed in different stages of COPD and is correlated positively with lung function parameters.
Conclusion: From these findings we conclude that as the severity increases there is decrease in alpha-1-antitrypsin resulting in concomitant increase in neutrophil elastase activity causing imbalance between protease-antiprotease in COPD patients and this imbalance is associated with impairment of lung function. The neutrophil elastase/ α-1-antitrypsin ratio can tell us the severity of the chronic obstructive pulmonary disease it may be in terms of increased fibrosis of the lung. Though the magnitude of neutrophil elatase/alpha-1-antitrypsin ratio is minute, still it can be a good marker of pulmonary function in term of severity of the COPD.
Downloads
References
Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006 Nov;3(11):e442.
(GOLD) Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease. 2013; February. Available at: http://www.goldcopd.org
Jindal SK, Aggarwal AN, Chaudhry K et al. A multicentric study on epidemiology of chronic obstructive pulmonary disease and its relationship with tobacco smoking and environmental tobacco smoke exposure. Indian J Chest Dis Allied Sci 2006; 48: 23-29.
Barnes PJ. Mediators of chronic obstructive pulmonary disease. Pharmacol Rev. 2004 Dec; 56(4):515-48.
Jadhav BS, Bradapurkar JS, Bhagwat VR, Bardapukar SJ. Evaluation of total serum alpha-1-antitrypsin and vitamin E in smoker and non-smoker chronic obstructive pulmonary disease patients. Biomedicine 2013;33(4): 520-525.
Rai R, Phadke M. Plasma antiprotease status in different respiratory disorders. The Internet Journal of Pulmonary Medicine.2006; 7(1):1-6.
Carrell RW, Jeppsson JO, Laurell CB, Brennan SO, Owen MC, Vaughan L, Boswell DR. Structure and variation of human alpha 1-antitrypsin. Nature. 1982 Jul 22;298(5872):329-34.
Carrell RW, Jeppsson JO, Vaughan L, Brennan SO, Owen MC, Boswell DR. Human alpha 1-antitrypsin: carbohydrate attachment and sequence homology. FEBS Lett. 1981 Dec 7;135(2):301-3.
Kalsheker N, Morley S, Morgan K. Gene regulation of the serine proteinase inhibitors alpha1-antitrypsin and alpha1-antichymotrypsin. Biochem Soc Trans 2002; 30(2):93-98.
Olsen GN, Harris JO, Castle JR, Waldman RH, Karmgard HJ: Alpha-1-antitrypsin content in the serum, alveolar macrophages, and alveolar lavage fluid of smoking and nonsmoking normal subjects. J Clin Invest 1975; 55(2):427-430.
Perlmutter DH, Cole FS, Kilbridge P, Rossing TH, Colten HR. Expression of the alpha 1-proteinase inhibitor gene in human monocytes and macrophages. Proc Natl Acad Sci U S A. 1985 Feb;82(3):795-9.
Stockley RA. Proteases and antiproteases. Novartis Found Symp. 2001;234:189-99; discussion 199-204.
Bühling F, Groneberg D, Welte T. Proteases and their role in chronic inflammatory lung diseases. Curr Drug Targets. 2006 Jun;7(6):751-9.
Lang MR, Fiaux GW, Gillooly M, Stewart JA, Hulmes DJ, Lamb D. Collagen content of alveolar wall tissue in emphysematous and non-emphysematous lungs. Thorax. 1994 Apr;49(4):319-26.
Lee WL, Downey GP. Leukocyte elastase : Physiological function and role in acute lung injury. Am. J. Respir. Crit. Care Med. 2001; 164(5): 896-904.
Perlmutter DH, Pierce JA. The alpha 1-antitrypsin gene and emphysema. Am J Physiol. 1989; 257(4 Pt 1): L147-62.
Carp H, Janoff A. Possible mechanism of emphysema in smokers in vitro suppression of serum elastase-inhibitory capacity by fresh cigarette smoke and its prevention by antioxidants. Am. Rev. Respir. Dis. 1978; 118(3): 617-621. doi: https://doi.org/10.1164/arrd.1978.118.3.617.
Erickson JA, Jensen RA, Grenache DG. Enzyme linked immunosorbent assay for the determination of alpha-1-antitrypsin in serum and stool. Immuchrome Gmbh. The Journal of Applied Laboratory Medicine. 2016; 1(1):60-66. doi: https://doi.org/10.1373/jalm.2016.020198 Published July 201.
Enzyme linked immunosorbent assay for the determination of neutrophil elastase in serum. Wuhan EIAab Sci. Co. Ltd. Available at : http://www.eiaab.com/entries/steps/ELISA%20Kit/10099_EIAAB/Human
Hatman TE, Tazeloar HD, Swensen SJ, Muller NL. Cigarette smoking: CT and pathological findings of associated pulmonary disease. Radiographics 1997;17(2): 377-390.
John ER, Aalt B, Ida L. Oxidative stress in chronic obstructive pulmonary disease. Oxidative stress study group. Am. J. Respir. Crit. Care. Med. 1997; 156(2 pt 1): 341-57. doi: https://doi.org/10.1164/ajrccm.156.2.9611013.
Daphne CR, James RJ, Nell H, Mae MS, Elvism I. Diagnostic value of post bronchodilator pulmonary function testing to distinguish between stable moderate to severe COPD and asthma. Intr. J. Chron. Obstruct.Pulmon Dis. 2008; 3(4): 693-699.
Hogg JC, Chu F, Utokaparch S, Woods R, Elliot WM, Buzatu L, Cherniack RM, Rogers RM, Sciurba FC, Coxson HO, Pare PD. The nature of small airway obstruction in COPD. N Eng J Med. 2004; 350:2645-2653. doi: https://doi.org/10.1056/NEJMoa032158.
Saetta M, Turato G, Maestrelli P, Mapp CE, Fabrri LM. Cellular and structural bases of chronic obstructive pulmonary disease. Am. J. Respi. Crit. Care Med. 2001; 163(6):1304-9. doi: https://doi.org/10.1164/ajrccm.163.6.2009116.
Fick RB, Naegel GP, Aquier S, Wood RE, Gee JBL, Reynold HY. Proteins of cystic fibrosis respiratory tract: fragmented immunoglobulin G opsonic antibody causing defective opsophagocytosis. J. Clin. Invest. 1984;74(1): 236-248. doi: https://doi.org/10.1172/JCI111407.
Meyer KC, Lewandoski JR, Zimmerman JJ, Nunley D, Calhoun WJ, Dopico GA. Human neutrophil elastase and elastase/alpha1-antiprotease complex in cystic fibrosis : comparison with interstitial lung disease and evaluation of the effect of intravenously administered antibiotic therapy. Am Rev. Respir. Dis. 1991; 144 (3 pt 1):580-5. doi: https://doi.org/10.1164/ajrccm/144.3_Pt_1.580
Kodanma T, Yukioka H, Kato T, Kato N, Hato F, Kitagawa S. Neutrophil elastase as a predicting factor for development of acute lung injury. Internal Medicine. 2007; 46(11):699-94.
Moroy G, Alix AJ, Sapi J, Hornebeck W, Bourguet E. Neutrophil elastase as a target in lung cancer. Anticancer Agents Med Chem. 2012 Jul;12(6):565-79.
Borzi RM, Grigolo B, Meliconi R, Fasano L, Sturani C, Fabbri M, Porstmann J, Fancchini A. Elevated serum superoxide dismutase levels correlate with disease severity and neutrophil degranulation in idiopathic pulmonary fibrosis. Clin. Sci. (Lond) 1993; 85(3):353-9. doi: https://doi.org/10.1042/cs0850353.
Beatty K, Bieth J, Travis J. Kinetics of association of serine proteinases with native and oxidized alpha-1-proteinase inhibitor and alpha-1-antichymotrypsin. J Biol Chem. 1980 May 10;255(9):3931-4.
Taggart C, Cervantes-Laurean D, Kim G, McElvaney NG, Wehr N, Moss J, Levin RL. Oxidation of either methionine 351 or methionine 358 in α-1-antitrypsin causes loss of antineutrophil elastase activity. J. Biol. Chem.1980; 275(35):27258-65. doi: https://doi.org/10.1074/jbc.M004850200
Gadek JE, Pacht ER. The protease antiprotease balance within the human lung: implications for the pathogenesis of emphysema. Lung. 1990; 168 (1): 552-564. doi: https://doi.org/10.1007/BF02718178
Kilore-Smith TA, Dowdeswell RJ, Gaillard MC. Elastase binding capacity of α2-macroglobulin in plasma of patients with asthma or chronic obstructive pulmonary disease without α-1 protease inhibitor deficiency. Clinica Chimica Acta 1989; 185(1) :81-90. doi: https://doi.org/10.1016/0009-8981(89)90133-2
Denchev K, Radkov M, Lipcheva N. Changes in the level of alpha1-antitrypsin in bronchial asthma. Vutr Boles. 1977; 16(2):75-8.
Sohrab S, Petrusca DN, Lockett AD, Schweitzer KS, Rush NI, Gu Y, Kamocki K, Garrison J, Petrache I. Mechanism of alpha-1 antitrypsin endocytosis by lung endothelium. FASEB J. 2009 Sep; 23(9):3149-58. doi: https://doi.org/10.1096/fj.09-129304. Epub 2009 May 7.
Gaillard MC, Kilore-Smith TA, Nogueira C, Dunn D, Jenkins T, Fine B, Kllenbach J. Alpha-1-protease inhibitor in bronchial asthma: phenotypes and biochemical characteristics. Am Rev Respire Dis. 1992: 145(6):1311-5. doi: https://doi.org/10.1164/ajrccm/145.6.1311.


 OAI - Open Archives Initiative
OAI - Open Archives Initiative


