The relationship between blood sugar levels (glycosylated haemoglobin) and the risk of development of diabetic retinopathy
Abstract
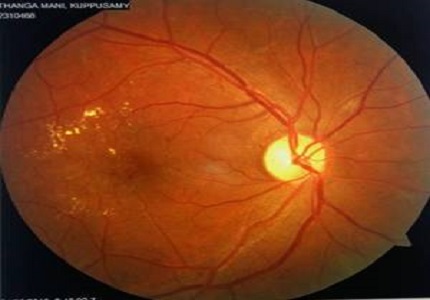
Introduction: Diabetic retinopathy is a major cause of visual impairment and blindness in India and with its early detection and timely treatment, the risk of visual loss can be reduced significantly. Onset and progression of retinopathy is determined by fasting and postprandial blood sugar levels and other risk factors such as duration of diabetes, hypertension, nephropathy, hypercholesterolemia and obesity. This study was done to evaluate the role of glycosylated hemoglobin and its association with the severity of retinopathy.
Materials and Methods: A total of 100 patients were included. This was a prospective study conducted over 1 year in Sri Ramachandra University, Chennai, India. Fundus examination was done using slit lamp biomicroscopy and indirect ophthalmoscopy on all patients. Ancillary investigations such as fundus fluorescein angiography and optical coherence tomography were performed. HbA1c was measured along with fasting, postprandial, lipid profile and urine sugars.
Results: High and uncontrolled levels of HbA1c were associated with maculopathy. A statistically significant difference (p < 0.01) was found in them. The retinopathy however was not related to HbA1C alone. The most frequent type of maculopathy noted was cystoid macular oedema and the level above which it occurred was 7% of HbA1C. Besides HbA1C, the other important factor that was associated with maculopathy was duration of diabetes mellitus.
Conclusion: HbA1c value >7.0% was significantly related with maculopathy. The severity of retinopathy is dependent on the level of blood sugar and duration of diabetes. Control of sugar levels is crucial to prevent diabetic retinopathy and its complications which can lead to irreversible complications in the eye.
Downloads
References
Krishnamurti U, Steffes MW. Glycohemoglobin: a primary predictor of the development or reversal of complications of diabetes mellitus. Clin Chem. 2001;47(7):1157-65.
Seema Abhjeet Kaveeshwar, John Cornwall. The current state of diabetes mellitus in India. Australas Med J. 2014; 7(1): 45-48. DOI: https://dx.doi.org/10.4066%2FAMJ.2013.1979.
Davis MD, Fisher MR, Gangnon RE, Barton F, Aiello LM, Chew EY, Ferris FL 3rd, Knatterud GL. Risk factors for high-risk proliferative diabetic retinopathy and severe visual loss: Early Treatment Diabetic Retinopathy Study Report #18. Invest Ophthalmol Vis Sci. 1998 Feb;39(2):233-52.
Klein R. The Diabetes Control and Complications Trial. Kertes C,ed. Clinical Trials in Ophthalmology: A Summary and Practice Guide. 1998.49-70.
Ciulla TA, Amador AG, Zinman B. Diabetic retinopathy and diabetic macular edema: pathophysiology, screening, and novel therapies. Diabetes Care. 2003 Sep;26(9):2653-64.
Klein R, Klein BEK, Moss SE, Davis MD, DeMets DL. Glycosylated haemoglobin predicts the incidence and progression of diabetic retinopathy. JAMA 1988 Nov; 260 (19): 2864–71.
Lachin JM, Genuth S, Nathan DM, Zinman B, Rutledge BN; DCCT/EDIC Research Group. Effect of glycemic exposure on the risk of microvascular complications in the diabetes control and complications trial--revisited. Diabetes. 2008 Apr;57(4):995-1001. doi: https://doi.org/10.2337/db07-1618. Epub 2008 Jan 25.
Klein R, Klein BE, Magli YL, Brothers RJ, Meuer SM, Moss SE, Davis MD. An alternative method of grading diabetic retinopathy. Ophthalmology 1986 Sep; 93(9):1183–7.
Agarwal S, Raman R, Paul PG, Rani PK, Uthra S, Gayathree R, McCarty C, Kumaramanickavel G, Sharma T. Sankara Nethralaya-Diabetic Retinopathy Epidemiology and Molecular Genetic Study (SN-DREAMS 1): study design and research methodology. Ophthalmic Epidemiol. 2005 Apr;12(2):143-53.
Tapp RJ, Tikellis G, Wong TY, Harper CA, Zimmet PZ, Shaw JE; Australian Diabetes Obesity and Lifestyle Study Group. Longitudinal association of glucose metabolism with retinopathy: results from the Australian Diabetes Obesity and Lifestyle (AusDiab) study. Diabetes Care. 2008 Jul;31(7):1349-54. doi: https://doi.org/10.2337/dc07-1707. Epub 2008 Apr 14.
Brown JB, Pedula KL, Summers KH. Diabetic retinopathy: contemporary prevalence in a well-controlled population. Diabetes Care. 2003 Sep;26(9):2637-42.
Anitha B, Sampathkumar R, Balasubramanyam M, Rema M. Advanced glycation index and its association with severity of diabetic retinopathy in type 2 diabetic subjects. J Diabetes Complications. 2008 Jul-Aug;22(4):261-6. doi: https://doi.org/10.1016/j.jdiacomp.2007.05.005. Epub 2008 Apr 16.
Schorr SG, Hammes HP, Müller UA, Abholz HH, Landgraf R, Bertram B. The Prevention and Treatment of Retinal Complications in Diabetes. Dtsch Arztebl Int. 2016 Dec 2;113(48):816-823. doi: https://dx.doi.org/10.3238%2Farztebl.2016.0816.
Liew G, Wong VW, Ho IV. Mini –Review: Changes in the Incidence of and Progression to Proliferative and Sight-Threatening Diabetic Retinopathy Over the Last 30 Years. Ophthalmic Epidemiol. 2017 Jan 19: 1-8.doi: https://doi.org/10.1080/09286586.2016.1259638.
Akil H, Buluş AD, Andiran N, Alp MN. Ocular manifestations of Type 1 diabetes mellitus in pediatric population. Indian J Ophthalmol. 2016 Sep;64(9):654-658. doi: https://dx.doi.org/10.4103%2F0301-4738.194336.
Roy S, Kern TS, Song B, Stuebe C. Mechanistic Insights into Pathological Changes in the Diabetic Retina: Implications for Targetng Diabetic Retinopathy. Am J Pathol. 2017 Jan; 187(1): 9-19. doi: https://doi.org/10.1016/j.ajpath.2016.08.022.
Amin ZA, Islam QU, Mehboob MA. Comparison of serum lipid profile in Type-2 Diabetics with and without retinopathy in Pakistani population. Pak J Med Sci. 2016 Nov-Dec;32(6):1349-1353. doi: https://doi.org/10.12669/pjms.326.11056.
Umayahara Y, Fujita Y, Watanabe H, Kasai N, Fujiki N, Hatazaki M, Koga M. Association of glycated albumin to HbA1c ratio with diabetic retinopathy but not diabetic nephropathy in patients with type 2 diabetes. Clin Biochem. 2016; Dec 5. pii: S0009-9120(16)30390-3. doi: https://doi.org/10.1016/j.clinbiochem.2016.11.032.


 OAI - Open Archives Initiative
OAI - Open Archives Initiative


