Evaluation of enzyme immunoassay based on detection of pLDH antigen for the diagnosis of malaria
Abstract
Introduction: Timely diagnosis of malaria is a challenge in most endemic areas due to lack of resources. The methods most commonly used are microscopy, regarded as the gold standard, and rapid dipstick tests (RDT) which detect antigens in blood. Enzyme-Linked Immuno Sorbent Assay (ELISA) based tests are fast and easy to perform especially when large number of samples have to be tested. p-LDH is a highly sensitive marker of malaria in blood The present study was done to assess the diagnostic performance of a p-LDH based ELISA on samples from clinically suspected malaria patients.
Methods: We tested the sensitivity and specificity of a pLDH based, commercially available ELISA kit on both microscopy positive and negative samples. Microscopy was done for all suspected malaria patients and of these 146 samples (73 positive and 73 negative) were tested by the ErbaLisa PAN (LDH) malaria ELISA kit as well SD Bioline malaria antigen test (RDT) based on detection of both HRP-2 and p-LDH common to all four species.
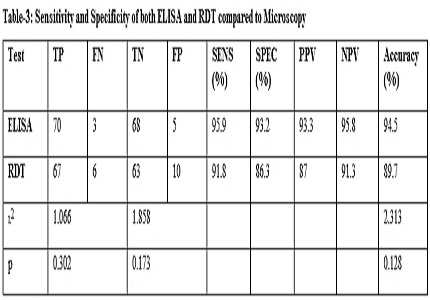
Results: The sensitivity of Elisa was 95.9% while specificity was 93.2 % compared to gold standard microscopy while RDTs had 91.8 % sensitivity and 86.3 % specificity. All 67 samples positive by both microscopy and RDT were also positive by ELISA.
Conclusion: p-LDH based ELISA promises to be a cost effective and reliable option for diagnosis of malaria in endemic areas like India.
Downloads
References
WHO: WORLD MALARIA REPORT 2015 Country profile India 2015. [http://www.who.int/malaria/publications/country-profiles/profile_ind_en.pdf?ua=1] last accessed on 10th October, 2015.
Tangpukdee N, Duangdee C, Wilairatana P, Krudsood S. Malaria diagnosis: a brief review. Korean J Parasitol. 2009 Jun;47(2):93-102. doi: https://doi.org/10.3347/kjp.2009.47.2.93. Epub 2009 May 26.
Zurovac D, Midia B, Ochola SA, English M, Snow RW. Microscopy and outpatient malaria case management among older children and adults in Kenya. Trop Med Int Health. 2006 Apr;11(4):432-40.doi: https://doi.org/10.1111/j.1365-3156.2006.01587.x.
Britton S, Cheng Q, McCarthy JS. Novel molecular diagnostic tools for malaria elimination: a review of options from the point of view of high-throughput and applicability in resource limited settings. Malar J. 2016 Feb; 15: 88. doi: https://doi.org/10.1186/s12936-016-1158-0.
Kifude CM, Rajasekariah HG, Sullivan DJ, Stewart VA, Angov E, Martin SK, Diggs CL, N. Waitumbi JN. Enzyme-Linked Immunosorbent Assay for Detection of Plasmodium falciparum Histidine-Rich Protein 2 in Blood, Plasma, and Serum. Clin Vaccine Immunol. 2008 Jun;15(6):1012-8. doi: https://doi.org/10.1128/CVI.00385-07.
Wongsrichanalai C, Barcus MJ, Muth S, Sutamihardja A, Wernsdorfer WA. A Review of Malaria Diagnostic Tools: Microscopy and Rapid Diagnostic Test (RDT) Am. J. Trop. Med. Hyg., 77(Suppl 6); 2007:119–27.doi: https://doi.org/10.4269/ajtmh.2007.77.119.
Bell DR, Wilson DW, Martin LB, 2005. False-positive results of a Plasmodium falciparum histidine-rich protein 2-detecting malaria rapid diagnostic test due to high sensitivity in a community with fluctuating low parasite density. Am J Trop Med Hyg 73: 199–203.doi: https://doi.org/10.4269/ajtmh.2005.73.199.
Moges B, Amare B, Belyhun Y, Tekeste Z, Gizachew M, Workineh M, Gebrehiwot A, Woldeyohannes D, Mulu A, Kassu A. Comparison of CareStart™ HRP2/pLDH COMBO rapid malaria test with light microscopy in north-west Ethiopia. Malar J. 2012 Jul;11:234. doi: https://doi.org/10.1186/1475-2875-11-234.
Abba K, Kirkham AJ, Olliaro PL, Deeks JJ, Donegan S, Garner P, Takwoingi Y. Rapid diagnostic tests for diagnosing uncomplicated non-falciparum or Plasmodium vivax malaria in endemic countries. Cochrane Database Syst Rev. 2014 Dec 18; (12): 1–195. doi: https://doi.org/10.1002/14651858.CD011431.
Lee JH, Jang JW, Cho CH, Kim JY, Han ET, Yun SG, Lim CS. False-positive results for rapid diagnostic tests for malaria in patients with rheumatoid factor. J Clin Microbiol. 2014 Oct;52(10):3784-7. doi: https://doi.org/10.1128/JCM.01797-14. Epub 2014 Jul 23.
Akotet BMK, Nkare CA, Mbouoronde OC, Mawili-Mboumba DP. Performances of SD Bioline Malaria Ag-P.F/Pan RDT for the Diagnosis of Malaria in Febrile Patients Living In Gabon, Central Africa. Malar Chemoth Cont Elimination.2014; 3:125. doi: https://doi.org/10.4172/2090-2778.1000125.
Noedl H, Yingyuen K, Laoboonchai A, Fukuda M, Sirichaisinthop J, Miller RS. Sensitivity and specificity of an antigen detection ELISA for malaria diagnosis. Am J Trop Med Hyg. 2006 Dec;75(6):1205-8.doi: https://doi.org/10.4269/ajtmh.2006.75.1205.
Thongdee P, Chaijaroenkul W, Kuesap J, Na-Bangchang K. Nested-PCR and a new ELISA-based NovaLisa test kit for malaria diagnosis in an endemic area of Thailand. Korean J Parasitol. 2014 Aug;52(4):377-81. doi: https://doi.org/10.3347/kjp.2014.52.4.377. Epub 2014 Aug 29.
Oh JS, Kim JS, Lee CH, Nam DH, Kim SH, Park DW, Lee CK, Lim CS, Park GH. Evaluation of a malaria antibody enzyme immunoassay for use in blood screening. Mem Inst Oswaldo Cruz. 2008 Feb;103(1):75-8. Epub 2008 Jan 31.doi: https://doi.org/10.1590/s0074-02762008005000008.
Kim J, Jang JW, Kim JY, Oh DJ, Lim CS. Combined Use of Malaria Antigen and Antibody Enzyme-Linked Immunosorbent Assay for Blood Screening of Plasmodium vivax in the Republic of Korea. Med Princ Pract. 2016;25(3):212-8. doi: https://doi.org/10.1159/000444144.


 OAI - Open Archives Initiative
OAI - Open Archives Initiative


