The application of gene xpert for the diagnosis of mycobacterium tuberculosis and MDR TB in Kota region
Abstract
Background: Improved diagnosis of tuberculosis is a global priority for tuberculosis control which requires early case-detection. MDR-TB poses formidable challenges due to the complex requirements for diagnosis and treatment and so enhancement in the capacity to diagnose MDR TB is required. The objective of the study is to assess the performance of GeneXpert MTB/RIF as an initial diagnostic tool for diagnosis of Mycobacterium tuberculosis and MDR TB in kota region.
Methods: Total 330 patients of all age groups having either pulmonary or extrapulmonary tuberculosis were included in the study with study period from February 2016 to July 2016 and samples collected were subjected to Gene Xpert.
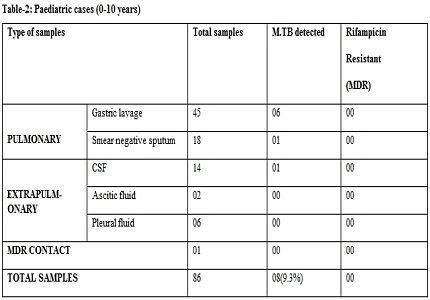
Results: Mycobacterium tuberculosis was reported in 102(30.90%) cases by Gene Xpert and Rifampicin Resistance was detected in 23(22.54%) out of these 102 cases.
Conclusion: The implementation of the Xpert MTB/RIF assay could dramatically improve the rapid diagnosis of tuberculosis, especially in cases with suspicion of MDR and smear negative TB.
Downloads
References
TB India 2016 Revised National Tuberculosis Control Programme, Annual Status Report.https://www.tbcindia.gov.in/WriteReadData/l892s/3608495828TB%20India%202016_Part1.pdf.
Tuberculosis. WHO Global Tuberculosis Report 2014. http://www.who.int/tb/ publications/factsheet_global.pdf.
Automated Real-Time Nucleic Acid Amplification Technology for Rapid and Simultaneous Detection of Tuberculosis and Rifampicin Resistance: Xpert MTB/ RIF Assay for the Diagnosis of Pulmonary and Extrapulmonary TB in Adults and Children: Policy Update. Geneva: World Health Organization;Issued date 2013. https://apps.who.int/iris/bitstream/handle/10665/112472/9789241506335_eng.pdf?sequence=1&isAllowed=y.
S teingart KR, Schiller I, Horne DJ, Pai M, Boehme CC, Dendukuri N. Xpert® MTB/RIF assay for pulmonary tuberculosis and rifampicin resistance in adults. Cochrane Database Syst Rev. 2014 Jan 21;(1):CD009593. doi: https://doi.org/10.1002/14651858.CD009593.pub2.
Small PM, Pai M. Tuberculosis diagnosis--time for a game change. N Engl J Med. 2010 Sep 9;363(11):1070-1. doi: https://doi.org/10.1056/NEJMe1008496. Epub 2010 Sep 1.
Weyer K, Mirzayev F, Migliori GB, Van Gemert W, D'Ambrosio L, Zignol M, Floyd K, Centis R, Cirillo DM, Tortoli E, Gilpin C, de Dieu Iragena J, Falzon D, Raviglione M. Rapid molecular TB diagnosis: evidence, policy making and global implementation of Xpert MTB/RIF. Eur Respir J. 2013 Jul;42(1):252-71. doi: https://doi.org/10.1183/09031936.00157212. Epub 2012 Nov 22.
Xpert MTB/RIF for people living with HIV. World Health Organization October 2014.http://www.who.int/tb/challenges/hiv/Xpert_TBHIV_Information_Note_final. Assesed on 13 Nov;2014.
Walusimbi S, Bwanga F, De Costa A, Haile M1, Joloba M, Hoffner S. Meta-analysis to compare the accuracy of GeneXpert, MODS and the WHO 2007 algorithm for diagnosis of smear-negative pulmonary tuberculosis. BMC Infect Dis. 2013 Oct 30;13:507. doi: https://doi.org/10.1186/1471-2334-13-507.
Theron G, Peter J, Dowdy D, Langley I, Squire SB, Dheda K. Do high rates of empirical treatment undermine the potential effect of new diagnostic tests for tuberculosis in high-burden settings? Lancet Infect Dis. 2014 Jun;14(6):527-32. doi: https://doi.org/10.1016/S1473-3099(13)70360-8. Epub 2014 Jan 15.
Guidance document for use of Catridge Based-Nucleic Acid Amplification Test (CB- NAAT) under Revised National TB Control Programme (RNTCP) issued central TB division, directorate general of health services september2013.
Geneva: World Health Organization; 2013. WHO Guidelines Approved by the Guidelines Review Committee. Automated Real-Time Nucleic Acid Amplification Technology for Rapid and Simultaneous Detection of Tuberculosis and Rifampicin Resistance: Xpert MTB/RIF Assay for the Diagnosis of Pulmonary and Extrapulmonary TB in Adults and Children: Policy Update.
World Health Organisation. Guidance for national tuberculosis programmes on the management of tuberculosis in children second edition. Geneva, Switzerland;2014. http://apps.who.int/iris/bitstream/10665/112360/1/9789241548748_eng.pdf. Accessed 8th September 2014.
Swaminathan S, Rekha B. Pediatric tuberculosis: global overview and challenges. Clin Infect Dis. 2010 May 15;50 Suppl 3:S184-94. doi: https://doi.org/10.1086/651490.
Kelynack T. Tuberculosis in Infancy and Children. New York: William wood and Co; 1908.
"Frequently asked questions on Xpert MTB/RIF assay" Retrieved on 12 June 2012, http://www.medscape.com/viewarticle/745030_2
D. W. Dowdy, A. Cattamanchi, K. R. Steingart, and M. Pai, “Is scale-up worth it? Challenges in economic analysis of diagnostic tests for tuberculosis,” PLoS Medicine, vol. 8, no. 7, Article ID e1001063, 2011. Published: July 26, 2011. http://dx.doi.org/10.1371/journal.pmed.1001063.
Vassall A, van Kampen S, Sohn H, Michael JS, John KR, den Boon S, Davis JL, Whitelaw A, Nicol MP, Gler MT, Khaliqov A, Zamudio C, Perkins MD, Boehme CC, Cobelens F. Rapid diagnosis of tuberculosis with the Xpert MTB/RIF assay in high burden countries: a cost-effectiveness analysis. PLoS Med. 2011 Nov;8(11):e1001120. doi: https://doi.org/10.1371/journal.pmed.1001120. Epub 2011 Nov 8.
Lawn SD, Zumla AI. Diagnosis of extrapulmonary tuberculosis using the Xpert(®) MTB/RIF assay. Expert Rev Anti Infect Ther. 2012 Jun;10(6):631-5. doi: https://doi.org/10.1586/eri.12.43.
Lawn SD, Churchyard G. Epidemiology of HIV-associated tuberculosis. Curr Opin HIV AIDS. 2009 Jul;4(4):325-33. doi: https://doi.org/10.1097/COH.0b013e32832c7d61.
Abdool Karim SS, Churchyard GJ, Abdool KQ, Lawn SD. HIV infection and tuberculosis in South Africa: an urgent need to escalate the public health response. Lancet 2009;374:921–933. doi: https://doi.org/10.1016/S0140-6736(09)60916-8. Epub 2009 Aug 24.
Reid MJ, Shah NS. Approaches to tuberculosis screening and diagnosis in people with HIV in resource-limited settings. Lancet Infect Dis. 2009 Mar;9(3):173-84. doi: https://doi.org/10.1016/S1473-3099(09)70043-X.
Getahun H, Harrington M, O'Brien R, Nunn P. Diagnosis of smear-negative pulmonary tuberculosis in people with HIV infection or AIDS in resource-constrained settings: informing urgent policy changes. Lancet. 2007 Jun 16;369(9578):2042-9.doi: https://doi.org/10.1016/s0140-6736(07)60284-0.
Hargreaves NJ, Kadzakumanja O, Whitty CJ, Salaniponi FM, Harries AD, Squire SB. Smear-negative pulmonary tuberculosis in a DOTS programme: poor outcomes in an area of high HIV seroprevalence. Int J Tuberc Lung Dis 2001;5:847–854.
Banda H, Kang’ombe C, Harries AD, Nyangulu DS, Whitty CJ, Wirima JJ, Salanipon FM, Maher D, Nunn P. Mortality rates and recurrent rates of tuberculosis in patient with smear-negative pulmonary tuberculosis and tuberculous pleural effusion who have completed treatment. Int J Tuberc Lung Dis. 2000 Oct;4(10):968-74.
Palmieri F, Girardi E, Pellicelli AM, Rianda A, Bordi E, Rizzi EB, Petrosillo N, Ippolito G. Pulmonary tuberculosis in HIV-infected patients presenting with normal chest radiograph and negative sputum smear. Infection. 2002 Apr;30(2):68-74.doi: https://doi.org/10.1007/s15010-002-2062-9.
Pablos-Méndez A, Sterling TR, Frieden TR. The relationship between delayed or incomplete treatment and all-cause mortality in patients with tuberculosis. JAMA. 1996 Oct 16;276(15):1223-8.doi: https://doi.org/10.1001/jama.1996.03540150025026.
Gandhi NR, Shah NS, Andrews JR, Vella V, Moll AP, Scott M, Weissman D, Marra C,Lalloo UG, Friedland GH. HIV coinfection in multidrug- and extensively drug-resistant tuberculosis results in high early mortality. Am J Respir Crit Care Med 2010 Jan 1;181(1):80-6. doi: https://doi.org/10.1164/rccm.200907-0989OC. Epub 2009 Oct 15.
Meintjes G, Rangaka MX, Maartens G, Rebe K, Morroni C, Pepper DJ, Wilkinson KA, Wilkinson RJ. Novel relationship between tuberculosis immune reconstitution.inflammatory syndrome and antitubercular drug resistance. Clin Infect Dis 2009 Mar 1;48(5):667-76. doi: https://doi.org/10.1086/596764.
Van Deun A, Barrera L, Bastian I, Fattorini L, Hoffmann H, Kam KM, Rigouts L, Rüsch-Gerdes S, Wright A. Mycobacterium tuberculosis strains with highly discordant rifampin susceptibility test results. J Clin Microbiol. 2009 Nov;47(11):3501-6. doi: https://doi.org/10.1128/JCM.01209-09. Epub 2009 Sep 16.
Ho J, Jelfs P, Sintchencko V. Phenotypically occult multidrug-resistant Mycobacterium tuberculosis: dilemmas in diagnosis and treatment. J Antimicrob Chemother. 2013 Dec;68(12):2915-20. doi: https://doi.org/10.1093/jac/dkt284. Epub 2013 Jul 9.
Somoskovi A, Deggim V, Ciardo D, Bloemberg GV. Diagnostic implications of inconsistent results obtained with the Xpert MTB/Rif assay in detection of Mycobacterium tuberculosis isolates with an rpoB mutation associated with low-level rifampin resistance. J Clin Microbiol. 2013 Sep;51(9):3127-9. doi: https://doi.org/10.1128/JCM.01377-13. Epub 2013 Jul 12.
Williamson DA, Roberts SA, Bower JE, Vaughan R, Newton S, Lowe O, Lewis CA, Freeman JT. Clinical failures associated with rpoB mutations in phenotypically occult multidrug-resistant Mycobacterium tuberculosis. Int J Tuberc Lung Dis. 2012 Feb;16(2):216-20. doi: https://doi.org/10.5588/ijtld.11.0178.


 OAI - Open Archives Initiative
OAI - Open Archives Initiative


