Diagnostic and prognostic role of Ki-67 and cytokeratin-5 expression in BPH and carcinoma prostate
Abstract
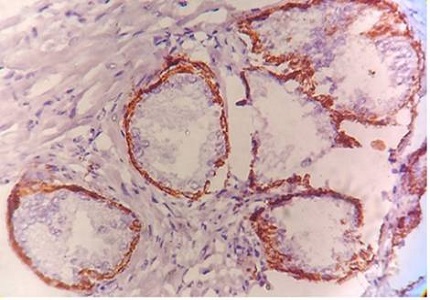
Background: Cytokeratin-5 which stains the basal cell layer of the of the prostatic glands and Ki-67 which is a proliferation marker and stains the proliferative population of the lesion has been employed as markers of two different categories viz. basal cell marker and proliferative markers respectively in various studies. Aim of this study was to evaluate the utility of cytokeratin-5 and Ki-67 in differentiating and diagnosing the benign lesion like Benign prostatic hyperplasia and malignant lesion i.e. Carcinoma Prostate.
Material and Methods: 90 cases of prostatic adenocarcinoma, BPH and prostatic inflammatory conditions were taken. Immunohistochemical staining for Ki67 and CK-5 was performed along with appropriate positive controls for each marker. Ki67 scoring classified as Negative, Low grade & High grade. Cytokeratin-5 scoring classified as Diffuse, Focal & Negative.
Results: Cytokeratin-5 expression was found positive in 96.71% cases of BPH. Out of these 78.68% cases were diffusely positive and 18.03% cases were focal positive. Expression of Cytokeratin-5 in Carcinoma Prostate was 40%, 6.66% and 0% in well, moderate and poorly differentiated carcinomas respectively, whereas no expression of the marker in 60%, 93.33% and 100% cases of well, moderate and poorly differentiated carcinomas respectively. Ki-67 showed low grade expression in mere 8.20% cases of BPH. In Carcinoma Prostate Ki67 expression was shown by 60%, 79% and 100% cases of well, moderate and poorly differentiated carcinomas respectively.
Conclusion: Diffuse Cytokeratin-5 expression was found in majority of BPH cases, while maximum Carcinoma prostate cases were negative. Ki-67 expression was found positively correlated with increasing Gleason’s grade in carcinoma prostate, while expression in benign lesion was not significant.
Downloads
References
2. Mathur SK, Gupta S, Marwah N, Narula A, Singh S, Arora B. Significance of mucin stain in differentiating benign and malignant lesions of prostate. Indian journal of pathology & microbiology. 2003 Oct;46(4):593-5.
3. Popkov VM, Maslyakova GN, Voronina ES. Immunohistochemical characteristics in diagnostics of prostate diseases. Russian Open Medical Journal. 2013;2(1):0109.
4. Wood DP, Honn KV, editors. The oncobiology of the prostate. Elsevier; 2000 Dec 20.
5. Munoz E, Gomez F, Paz JI, Casado I. Ki-67 immunolabeling in pre-malignant lesions and carcinoma of the prostate. Histological correlation and prognostic evaluation. European Journal of Histochemistry. 2009 Jun 26;47(2):123-8.
6. Sequeiros T, García M, Montes M, Oliván M, Rigau M, Colás E, de Torres I, Morote J, Reventós J, Doll A. Molecular markers for prostate cancer in formalin-fixed paraffin-embedded tissues. BioMed research international. 2013 Nov 25;2013.
7. Verma R, Gupta V, Singh J, Verma M, Gupta G, Gupta S, Sen R, Ralli M. Significance of p53 and ki-67 expression in prostate cancer. Urology annals. 2015 Oct;7(4):488. [PubMed]
8. Moll R, Franke WW, Schiller DL, Geiger B, Krepler R. The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors and cultured cells. cell. 1982 Nov 30;31(1):11-24.
9. Varadhachary GR, Abbruzzese JL, Lenzi R. Diagnostic strategies for unknown primary cancer. Cancer. 2004 May 1;100(9):1776-85.
10. Kanaji N, Bandoh S, Fujita J, Ishii T, Ishida T, Kubo A. Compensation of type I and type II cytokeratin pools in lung cancer. Lung Cancer. 2007 Mar 31;55(3):295-302. [PubMed]
11. Boran C, Kandirali E, Yilmaz F, Serin E, Akyol M. Reliability of the 34βE12, keratin 5/6, p63, bcl-2, and AMACR in the diagnosis of prostate carcinoma. InUrologic Oncology: Seminars and Original Investigations 2011 Dec 31 (Vol. 29, No. 6, pp. 614-623). Elsevier
12. Trpkov K, Bartczak-McKay J, Yilmaz A. Usefulness of cytokeratin 5/6 and AMACR applied as double sequential immunostains for diagnostic assessment of problematic prostate specimens. American journal of clinical pathology. 2009 Aug 1;132(2):211-20.
13. Diagnostic Immunohistochemistry: Theranostic and Genomic Applications: 4th Edition,David J Dabbs.immunohistochemistry staining Biogenx Immunohistochemistry kit manual.
14. Madani SH, Ameli S, Khazaei S, Kanani M, Izadi B. Frequency of Ki-67 (MIB-1) and P53 expressions among patients with prostate cancer. Indian Journal of Pathology and Microbiology. 2011 Oct 1;54(4):688.
15. Stapleton AM, Zbell P, Kattan MW, Yang G, Wheeler TM, Scardino PT, Thompson TC. Assessment of the biologic markers p53, ki‐67, and apoptotic index as predictive indicators of prostate carcinoma recurrence after surgery. Cancer. 1998 Jan 1;82(1):168-75.
16. Makarewicz R, Zyromska A, Andrusewicz H. Comparative analysis of biological profiles of Comparative analysis of biological profiles of Comparative analysis of biological profiles of benign prostate hyperplasia and prostate cancer as benign prostate hyperplasia and prostate cancer as potential diagnostic, prognostic and predictive potential diagnostic, prognostic and predictive indicators. ©Polish Society for Histochemistry and Cytochemistry Folia Histochem Cytobiol. 2011 10.5603/FHC.2011.0064 Vol. 49, No. 3, 2011 pp. 452–457.
17. Trpkov K, Bartczak-McKay J, Yilmaz A. Usefulness of cytokeratin 5/6 and AMACR applied as double sequential immunostains for diagnostic assessment of problematic prostate specimens. American journal of clinical pathology. 2009 Aug 1;132(2):211-20.
18. Goldstein NS. Immunophenotypic characterization of 225 prostate adenocarcinomas with intermediate or high Gleason scores. American journal of clinical pathology. 2002 Mar 1;117(3):471-7.
19. Adisa JO, Egbujo EC, Ibrahim B, Musa B, Madukwe J. Expression of some selected cytokeratins and Ki67 protein in prostatic tumor: can these be used as tumor markers.The Pan African Medical Journal. 2015;20:46. doi:10.11604/pamj.2015.20.46.3926.


 OAI - Open Archives Initiative
OAI - Open Archives Initiative


