Readdressing the role of therapeutic drug monitoring for antiepileptic drugs – A tertiary care hospital experience
Abstract
Background: Therapeutic drug monitoring is a beneficial tool to supervise patients when they do not respond to a therapeutic dose. Inter individual variability in the concentration of an antiepileptic drug that produces optimal therapeutic response is highly significant. Therefore, this retrospective study was taken up to study the inter relation between antiepileptic drug dosages, serum concentration sand clinical condition in the Indian patients.
Materials and Methods: This is a retrospective study, in which the data of the samples of adult patients of either gender, analyzed for Phenytoin, Valproate, Carbamazepine and Phenobarbitone were included. The samples were stratified based on dosage prescribed. The endpoints were to estimate the percentage of samples of each stratum having sub therapeutic, therapeutic and supra therapeutic concentrations.
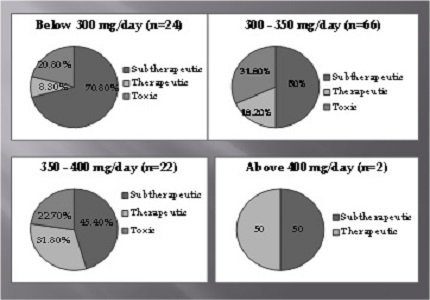
Results: Of the 134 samples included, 114 (85%) were analyzed for phenytoin, 9 for valproate, 7 for carbamazepine and 4 for phenobarbitone. Of the 114 samples analyzed for phenytoin, 61(53.5%) samples were having sub therapeutic concentrations, 22 samples (19.3%) had therapeutic concentrations and 31 samples (27.2%) had toxic concentrations. Among the 61 samples having sub therapeutic concentrations, 54.1% were prescribed dose of 300-350mg/day, 16.4% were on 350-400 mg/day and 1.6% were taking above 400mg/day. Of the total cases referred, 41.8 % had H/O of seizures and 30.6% presented with toxic symptoms.
Conclusion: This study demonstrated unpredictable inter individual variability in clinical response based on reference ranges. However, the relevance of individual reference concentrations for predicting outcomes can only be confirmed through adequately controlled randomized studies.
Downloads
References
2. Gross AS. Best practice in therapeutic drug monitoring. Br J Clin Pharmacol. 2001; 52 Suppl 1:5S-10S. [PubMed]
3. Snodgrass SR, Parks BR. Anticonvulsant blood levels: historical review with a pediatric focus. J Child Neurol. 2000 Nov; 15(11):734-46. [PubMed]
4. St Louis EK. Monitoring antiepileptic drugs: a level-headed approach. Curr Neuropharmacol. 2009 Jun; 7(2):115-9. doi: 10.2174/157015909788848938. [PubMed]
5. Schumacher GE, Barr JT, Browne TR, Collins JF. Test performance characteristics of the serum phenytoin concentration (SPC): the relationship between SPC and patient response. The Veterans Administration Epilepsy Cooperative Study Group. Ther Drug Monit. 1991 Jul; 13(4):318-24.
6. Kozer E, Parvez S, Minassian BA, Kobayashi J, Verjee Z, Koren G. How high can we go with phenytoin? Ther Drug Monit. 2002 Jun; 24(3):386-9. [PubMed]
7. Babaei A, Eslamai MH. Evaluation of therapeutic drug level monitoring of phenobarbital, phenytoin and carbamazepine in Iranian epileptic patients. Int J Clin Pharmacol Ther. 2007 Feb; 45(2):121-5. [PubMed]
8. Forooghipour M, Mohammadpour AH, MashhadianNV, Khayyat MH, Azarpajouh MR, Mokhber N et al. Iran J Basic Med Sci. Therapeutic Drug Monitoring of Valproic Acid in Patients with Mono therapy at Steady State. 2009 Autumn; 12(3-4):146-9.
9. Garg SK, Gupta MC, Handu SS, Bhargava VK. Indian Journal of Pharmacology. Therapeutic drug monitoring of antiepileptic drugs - A preliminary experience. 2000 Jan-Feb; 32 (1): 28-30.
10. Jannuzzi G, Cian P, Fattore C, Gatti G, Bartoli A, Monaco F et al. Epilepsia. A Multicenter Randomized Controlled Trial on the Clinical Impact of Therapeutic Drug Monitoring in Patients with Newly Diagnosed Epilepsy. The Italian TDM Study Group in Epilepsy. Epilepsia 2000 Feb; 41(2):222-30.
11. Dahiya K, Bansal P, Ghalaut VS, Dhankhar R, Ghalaut PS. Neurology Asia. Therapeutic drug monitoring for antiepileptic drugs using HPLC: An experience at a tertiary care hospital in India. 2010 Dec; 15(3):233-7. [PubMed]
12. Johannessen SI, Tomson T. ClinPharmacokinet. Pharmacokinetic variability of newer antiepileptic drugs: when is monitoring needed? 2006; 45(11):1061-75. [PubMed]
13. Johannessen SI, Tomson T. Pharmacokinetic variability of newer antiepileptic drugs: when is monitoring needed? Clin Pharmacokinet. 2006; 45(11):1061-75. [PubMed]
14. Tomson T, Dahl ML, Kimland E. Therapeutic monitoring of antiepileptic drugs for epilepsy. Cochrane Database Syst Rev. 2007 Jan 24; (1):CD002216.


 OAI - Open Archives Initiative
OAI - Open Archives Initiative


