Comparison of deferasirox and deferoxamine effects on iron overload in patients with blood transfusion-dependent β-thalassemia
Abstract
Introduction: Beta-thalassemias is autosomal recessive hematological disorder prevalent in the
Mediterranean area due to defects in synthesis of β chains of hemoglobin. The aim of present study was to compare the effects of deferasirox and deferoxamine on iron overload in patients with blood transfusion-dependent β-thalassemia major and intermedia.
Patients And Methods: This study involved 100 patients with known cases of β-thalassemia major or intermedia that has been treated with blood transfusion and iron chelators from January 2020 to December 2023. Serum ferritin, serum iron, serum total iron binding capacity were assessed in deferoxamine and deferasirox-treated patients.
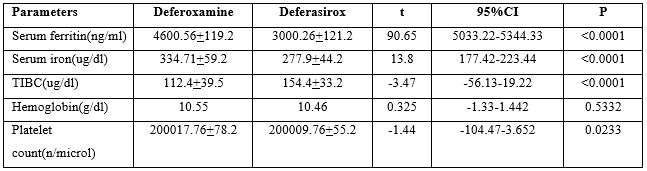
Results: In deferoxamine-treated patients, serum ferritin levels were high (4600.56 + 119.2ng/dL) compared to deferasirox-treated patients (3000.261 ± 121.2 ng/dL; P< 0.0001), also there were significant differences in serum iron and total iron-binding capacity (P< 0.0001) in deferasirox-treated patients compared to deferoxamine-treated patients.
Conclusion: This study indicated that deferasirox is more effective than deferoxamine regarding the iron overload in patients with blood transfusion-dependent β-thalassemia.
Downloads
References
2. Saetung R, Ongchai S, Charoenkwan P, Sanguansermsri T. Genotyping of beta thalassemia trait by high-resolution DNA melting analysis. Southeast Asian J Trop Med Public Health. 2013;44:1055–64.
3. Winichagoon P, Kumbunlue R, Sirankapracha P, Boonmongakol P, Fucharoen S. Discrimination of various thalassemia syndromes and iron deficiency and utilization of reticulocyte measurements in monitoring response to iron therapy. Blood Cells Mol Dis. 2015;54:336–41.
4. Moon SN, Han JW, Hwang HS, Kim MJ, Lee SJ, Lee JY, et al. Establishment of secondary iron overloaded mouse model: Evaluation of cardiac function and analysis according to iron concentration. PediatrCardiol. 2011;32:947–52.
5. Remacha ÁF, Arrizabalaga B, Villegas A, Durán MS, Hermosín L, de Paz R, et al. Evolution of iron overload in patients with low-risk myelodysplastic syndrome: Iron chelation therapy and organ complications. Ann Hematol. 2015;94:779–87.
6. Chiani M, Akbarzadeh A, Farhangi A, Mehrabi MR. Production of desferrioxamine B (Desferal) using corn steep liquor in Streptomyces pilosus. Pak J Biol Sci. 2010;13:1151–5.
7. Felice PA, Ahsan S, Donneys A, Deshpande SS, Nelson NS, Buchman SR. Deferoxamine administration delivers translational optimization of distraction osteogenesis in the irradiated mandible. PlastReconstr Surg. 2013;132:542e–8e.
8. Fisher SA, Brunskill SJ, Doree C, Chowdhury O, Gooding S, Roberts DJ. Oral deferiprone for iron chelation in people with thalassaemia. Cochrane Database Syst Rev. 2013;8:CD004839.
9. Bellanti F, Danhof M, Della Pasqua O. Population pharmacokinetics of deferiprone in healthy subjects. Br J Clin Pharmacol. 2014;78:1397–406.
10. Elalfy M, Wali YA, Qari M, Al Damanhouri G, Al-Tonbary Y, Yazman D, et al. Deviating from safety guidelines during deferiprone therapy in clinical practice may not be associated with higher risk of agranulocytosis. Pediatr Blood Cancer. 2014;61:879–84.
11. Chang HH, Lu MY, Peng SS, Yang YL, Lin DT, Jou ST, et al. The long-term efficacy and tolerability of oral deferasirox for patients with transfusion-dependent ß-thalassemia in Taiwan. Ann Hematol. 2015;94:1945–52.
12. Elalfy MS, Adly AM, Wali Y, Tony S, Samir A, Elhenawy YI. Efficacy and safety of a novel combination of two oral chelators deferasirox/deferiprone over deferoxamine/deferiprone in severely iron overloaded young beta thalassemia major patients. Eur J Haematol. 2015;95:411–20.
13. General Assembly of the World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. J Am Coll Dent. 2014;81:14–8.
14. Amah-Tariah FS, Ojeka SO, Dapper DV. Haematological values in pregnant women in Port Harcourt, Nigeria II: Serum iron and transferrin, total and unsaturated iron binding capacity and some red cell and platelet indices. Niger J Physiol Sci. 2011;26:173–8.
15. Cappellini MD, Porter J, El-Beshlawy A, Li CK, Seymour JF, Elalfy M, et al. Tailoring iron chelation by iron intake and serum ferritin: The prospective EPIC study of deferasirox in 1744 patients with transfusion-dependent anemias. Haematologica. 2010;95:557–66.
16. Cappellini MD. Exjade(R) (deferasirox, ICL670) in the treatment of chronic iron overload associated with blood transfusion. Ther Clin Risk Manag. 2007;3:291–9.
17. Vichinsky E, Torres M, Minniti CP, Barrette S, Habr D, Zhang Y, et al. Efficacy and safety of deferasirox compared with deferoxamine in sickle cell disease: Two-year results including pharmacokinetics and concomitant hydroxyurea. Am J Hematol. 2013;88:1068–73.
18. Nick H, Wong A, Acklin P, Faller B, Jin Y, Lattmann R, et al. ICL670A: Preclinical profile. Adv Exp Med Biol. 2002;509:185–203.
19. Cabantchik ZI, Breuer W, Zanninelli G, Cianciulli P. LPI-labile plasma iron in iron overload. Best Pract Res Clin Haematol. 2005;18:277–87.
20. Wood JC, Otto-Duessel M, Gonzalez I, Aguilar MI, Shimada H, Nick H, et al. Deferasirox and deferiprone remove cardiac iron in the iron-overloaded gerbil. Transl Res. 2006;148:272–80.
21. Wood JC, Cohen AR, Pressel SL, Aygun B, Imran H, Luchtman-Jones L, et al. Organ iron accumulation in chronically transfused children with sickle cell anaemia: Baseline results from the TWiTCH trial. Br J Haematol. 2016;172:122–30.
22. Saffari F, Mahyar A, Jalilolgadr S. Endocrine and metabolic disorders in ß-thalassemiamajor patients. Caspian J Intern Med. 2012;3:466–72.
Copyright (c) 2024 Author (s). Published by Siddharth Health Research and Social Welfare Society

This work is licensed under a Creative Commons Attribution 4.0 International License.


 OAI - Open Archives Initiative
OAI - Open Archives Initiative


