Monitoring And Evaluation Of Adverse Drug Reaction In Emergency Medicine Department
A Prospective Observational Study
Abstract
Background: Any deviation from the intended beneficial effect of a medication results in a drug related problem. Adverse drug reactions (ADRs) are negative consequences of drug therapy. It is the fourth to sixth leading cause of mortality in the United States of America.
Aims: To find out the proportion of medical emergency admissions that are secondary to Adverse Drug Reactions(ADRs).
Settings and Designs: An observational, prospective study conducted at the Emergency Medicine Department, at Tertiary Care Teaching Hospital for 12 months, daily from 9 am to 5pm.
Materials and Methods: Patients aged ≥ 18, who have given a written informed consent were included and patients not able to give willing consent and women presented with pregnancy were excluded in the study. The data was recorded in the case record form, The causality assessment was performed using WHO causality assessment scale. To determine the ADR severity, Modified Hartwig and Siegel scale was used.
Statistical Analysis: The statistical evaluation was done with the help of Statistical Package for Social Science (SPSS) version 21.0 manufactured by IBM (demo version) and Microsoft Excel 2016. p< 0.05 was considered as statistically significant.
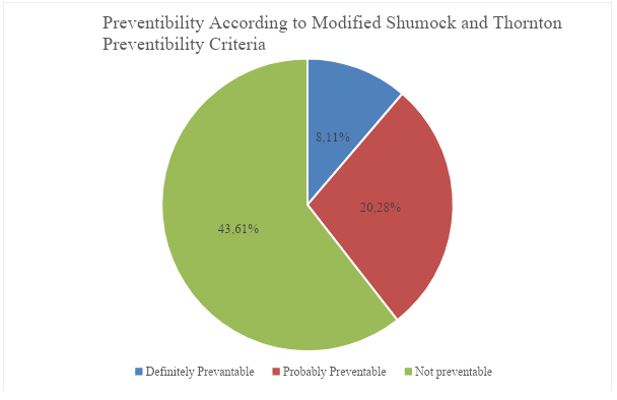
Results: Elderly patients were having higher incidence of ADRs. Among all drug groups, the highest incidence were antimicrobials and drugs acting on blood. Majority patients either recovered or were in a recovering phase. Most of the ADRs were not preventable.
Conclusions: Reporting of ADRs in a systematic way allows appropriate analysis and intervention which will improve the patient’s safety. Many ADRs could be preventable by avoiding certain drug/drug combinations, hospitalization, dose dependent side effects, appropriate individual dosing and applying the Antimicrobial Stewardship Programme.
Keywords: Adverse Drug Reactions(ADRs), Antimicrobials, Emergency Medicine Department
Downloads
References
2. Schurig AM, Böhme M, Just KS, Scholl C, Dormann H, Plank-Kiegele B, Seufferlein T, Gräff I, Schwab M, Stingl JC. Adverse Drug Reactions (ADR) and Emergencies. Dtsch Arztebl Int. 2018 Apr 13;115(15):251-258. doi: 10.3238/arztebl.2018.0251. PMID: 29735005; PMCID: PMC5949373
3. Geneva, Switzerland: World Health Organization. International drug monitoring: The role of hospitals. Technical report, 1969; 425.
4. Lazarou, J., B. H. Pomeranz, and P.N. Corey. Incidence of Adverse Drug Reactions in Hospitalized Patients: A Meta-analysis of Prospective Studies. JAMA 279, 1998; (15): 1200–05.
5. Pirmohamed, M. et al. Adverse Drug Reactions as Cause of Admission to Hospital: Prospective Analysis of 18,820 Patients. BMJ, 2004; 329: 15–19.
6. Y. Hassan1, R.J. Al-Ramahi, N.A. Aziz1 and R. Ghazali Adverse drug events in hospitalized patients with chronic kidney disease International Journal of Clinical Pharmacology and Therapeutics, 2010; 48(9): 571-576.
7. Shreya, Dr. (2018). Analysis of Adverse Drug Reactions in a Tertiary Care Emergency Medicine Department -Prevalence, Preventability and Reporting. International Journal of Basic & Clinical Pharmacology. 7. 1787. 10.18203/2319-2003.ijbcp20183490.
8. Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther 1981; 30(2): 239–45.
9. WHO-UMC causality assessment system. Available at http://www.whoumc.org/pdfs/Causality.pdf
10. Hartwig SC, Siegel J and Schneider PJ.Preventability and Severity Assessment in Reporting Adverse Drug reactions.American Journal of Hospital Pharmacy. 1992; 49: 2229-31.
11. Diana M R; Lennar et al. ADRs in a tertiary care emergency medicine ward- Prevalence, preventability and reporting P’LOS ONE ;sep,2016;1-14.
12. Rydberg DM et al. Adverse Drug Reactions in a Tertiary Care Emergency Medicine Ward - Prevalence, Preventability and Reporting. PLoS One. 2016 Sep 13;11(9):e0162948. doi: 10.1371/journal.pone.0162948. e Collection 2016.
13. Williamson J, Chopim JM. Adverse reactions to prescribed drug in elderly. A multicenric invigilation: Age- Ageing 1980:9;p-75-79.
14. [Nolan L,O’Malley.Prescribing for the elderly.Part 1:sensitivity of the elderly of adverse drug reactions.J.Am.Geriatric Soc. 1988:36;142-7]
15. Adegbite, B.R., Edoa, J.R., Schaumburg, F. et al. Knowledge and perception on antimicrobial resistance and antibiotics prescribing attitude among physicians and nurses in Lambaréné region, Gabon: a call for setting-up an antimicrobial stewardship program. Antimicrob Resist Infect Control 11, 44 (2022). https://doi.org/10.1186/s13756-022-01079-x
16. Kanjanarat P Winterstain AG et al. The nature of preventable adverse drug events in hospitals: A literature review. Am. J. Health syst. Pharm. 2003;60(17):1750-9.] [lerahenbuhl-melcher A, Schlienger R et al. Drug related problems in hospitals : a review of the recent literature. Drug saf.2007;30(5) 379-407.
17. Hakkarainen KM, Hedna K et al. Percentage of patients with preventable adverse drug reactions and preventability of adverse drug reactions- a meta-analysis. PLOS one 2012;7(3) 332-36.
18. Howard RL, Avery AJ et al. Which drug causes preventable admission to hospital? A systemic review, Br. J. Clin Pharmac. 2007;63(2) :136-47.
19. Lovborg H, Eriksson LR et al. A prospective analysis of preventability adverse drug reactions reported in Sweden. Eur. J. Clin. Pharmacol. 2012; 68(8): 1183-9.
20. James BC. Every defect a treasure : learning from adverse events in hospitals. Med.J.Aust,1997;166:484-2.
21. Raut A, Patel P et al. Preventability and predictability and seriousness of adverse drug reactions amongst medicine patients in a tertiary hospital. A prospective observational study. Int.J.Pharm.Chem.Science;2012,1(13):1293-99.
22. Amin S, Shah S, Desai M et al. An analysis of the adverse drug reactions in extremes of age group at tertiary care teaching hospital. Perspect.Clin.Res.2018;9:70-5.
23. Patel TK,Patel PH. Incidence of adverse drug reactions in Indian hospitals. A systemic review of prospective studies. Carr Drug Saf. 2016:11(2);120-36.
24. Sivanandy palanisamy, kottur sg arul kumaran, aiyalu rajasekaran, a study on assessment, monitoring and reporting of adverse drug reactions in indian hospital, Asian Journal of Pharmaceutical and Clinical Research, Vol 4, Issue 3, 2011, 112116.
Copyright (c) 2023 Author (s). Published by Siddharth Health Research and Social Welfare Society

This work is licensed under a Creative Commons Attribution 4.0 International License.


 OAI - Open Archives Initiative
OAI - Open Archives Initiative


