LYMPH NODE METASTASIS AFTER NEOADJUVANT CHEMOTHERAPY
DEFAULTER- TRIPLE NEGATIVE CARCINOMA WITH AXILLARY INVOLVEMENT RIGHT BREAST
Abstract
Neoadjuvant therapy refers to the systemic treatment of breast cancer prior to definitive surgical therapy (ie,
preoperative therapy). Neoadjuvant chemotherapy is offered to patients with locally advanced breast cancer and also
those breast cancer patients who may benefit from size reduction before conservation therapy. Response to neoadjuvant
chemotherapy is evaluated by the change in tumor size from pretreatment clinical and/or radiologic measurement to
post-treatment status. The spectrum of response to neoadjuvant chemotherapy varies from complete response, partial
response, to non-response. This concept is the same in breast tumors as well as axillary lymph nodes. The presented
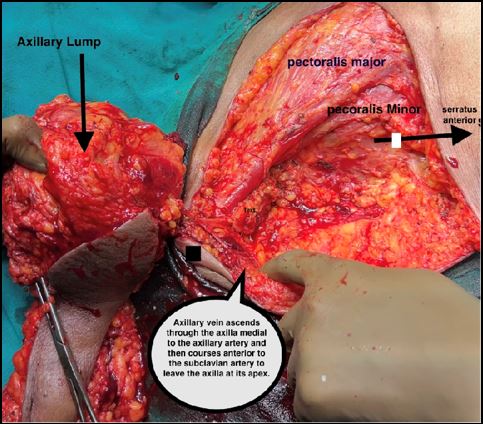
case is a known case of Triple Negative Invasive Ductal carcinoma with Axillary involvement Right Breast since
November, 2020 and had undergone Neoadjuvant Chemotherapy till February 2021, followed by surgical intervention
in October 2022.
Downloads
References
Shigematsu, H., Ozaki, S., Yasui, D., & Hirata, T. (2017). A case report of locally advanced triple negative breast cancer showing pathological complete response to weekly paclitaxel with bevacizumab treatment following disease progression during anthracycline-based neoadjuvant chemotherapy. International Journal of Surgery Case Reports, 39, 293-296. https://doi.org/10.1016/j.ijscr.2017.08.055.
von Minckwitz G., Raab G., Caputo A. Doxorubicin with cyclophosphamide followed by docetaxel every 21 days compared with doxorubicin and docetaxel every 14 days as preoperative treatment in operable breast cancer: the GEPARDUO study of the German Breast Group. J. Clin. Oncol. 2005;23(April (12)):2676–2685. [PubMed] [Google Scholar]
Sikov W.M., Berry D.A., Perou C.M. Impact of the addition of carboplatin and/or bevacizumab to neoadjuvant once-per-week paclitaxel followed by dose-dense doxorubicin and cyclophosphamide on pathologic complete response rates in stage II to III triple-negative breast cancer: CALGB 40603 (Alliance) J. Clin. Oncol. 2015;33(January (1)):13–21.
Iwata H, Sato N, Masuda N, Nakamura S, Yamamoto N, Kuroi K, Kurosumi M, Tsuda H, Akiyama F, Ohashi Y, Toi M. Docetaxel followed by fluorouracil/epirubicin/cyclophosphamide as neoadjuvant chemotherapy for patients with primary breast cancer. Jpn J Clin Oncol. 2011 Jul;41(7):867-75. doi: 10.1093/jjco/hyr081. PMID: 21719750.
Sanford RA, Lei X, Barcenas CH, et al. Impact of Time from Completion of Neoadjuvant Chemotherapy to Surgery on Survival Outcomes in Breast Cancer Patients. Ann Surg Oncol 2016;23:1515-21. 10.1245/s10434-015-5020-3 [PubMed] [CrossRef] [Google Scholar]
Brenton JD, Carey LA, Ahmed AA, Caldas C. Molecular classification and molecular forecasting of breast cancer: ready for clinical application? J Clin Oncol. 2005 Oct;23(29):7350–7360. [PubMed] [Google Scholar] [Ref list]
Yoo TK, Moon HG, Han W, Noh DY. Time interval of neoadjuvant chemotherapy to surgery in breast cancer: how long is acceptable? Gland Surg. 2017 Feb;6(1):1-3. doi: 10.21037/gs.2016.08.06. PMID: 28210546; PMCID: PMC5293647.
Liedtke C, Mazouni C, Hess KR, André F, Tordai A, Mejia JA, Symmans WF, Gonzalez-Angulo AM, Hennessy B, Green M, Cristofanilli M, Hortobagyi GN, Pusztai L. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J Clin Oncol. 2008 Mar 10;26(8):1275-81. doi: 10.1200/JCO.2007.14.4147. Epub 2008 Feb 4. PMID: 18250347.
Vandergrift JL, Niland JC, Theriault RL, Edge SB, Wong YN, Loftus LS, Breslin TM, Hudis CA, Javid SH, Rugo HS, Silver SM, Lepisto EM, Weeks JC. Time to adjuvant chemotherapy for breast cancer in National Comprehensive Cancer Network institutions. J Natl Cancer Inst. 2013 Jan 16;105(2):104-12. doi: 10.1093/jnci/djs506. Epub 2012 Dec 21. PMID: 23264681; PMCID: PMC3611850.
Copyright (c) 2023 Author (s). Published by Siddharth Health Research and Social Welfare Society

This work is licensed under a Creative Commons Attribution 4.0 International License.


 OAI - Open Archives Initiative
OAI - Open Archives Initiative


