Assessment of Promoter hypermethylation of APC and BRCA1 in endometrial cancer.
Abstract
Introduction: Endometrial cancer is one of the most common cancers in women worldwide. The underlying cause of endometrial tumorigenesis remains elusive. Several genetic and epigenetic alterations are known to be involved in the carcinogenesis of endometrial carcinoma. One important and early epigenetic alteration that is attributed to endometrial carcinoma is the aberrant promoter hypermethylation of gene promoters. In this study, we have assessed the aberrant promoter hypermethylation of APC and BRCA1 in 78 endometrial cancer samples.
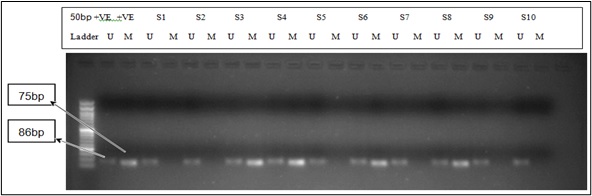
Methods: Histologically confirmed tumour tissue samples were obtained post-surgery and DNA was extracted. The DNA was subjected to sodium bisulfite conversion and used as a template for a polymerase chain reaction. The PCR was performed using a nested PCR followed by methylation specific PCR.
Results: A 33.33% and 46.15% methylation frequency was observed for APC and BRCA1 genes respectively. A higher percentage of methylation was observed in stage IV for APC (66.66%) and in stage II for BRCA1 (88.88%).
Conclusion: Aberrant promoter hypermethylation is an early event in endometrial carcinoma and can serve as a useful molecular marker for diagnosis and prognosis of the disease along with existing screening modalities.
Downloads
References
Banno K, Kisu I, Yanokura M, Masuda K, Kobayashi Y, Ueki A, et al. Endometrial Cancer and Hypermethylation: Regulation of DNA and MicroRNA by Epigenetics. Biochem Res Int. 2012;2012:738274. doi: 10.1155/2012/738274.
Huo X, Sun H, Cao D, Yang J, Peng P, Yu M, Shen K. Identification of prognosis markers for endometrial cancer by integrated analysis of DNA methylation and RNA-Seq data. Sci Rep. 2019 Jul 9;9(1):9924. doi: 10.1038/s41598-019-46195-8.
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016 Jan-Feb;66(1):7-30. doi: 10.3322/caac.21332.
Vrba L, Futscher BW. A suite of DNA methylation markers that can detect most common human cancers. Epigenetics. 2018;13(1):61-72. doi: 10.1080/15592294.2017.1412907.
Tao MH, Freudenheim JL. DNA methylation in endometrial cancer. Epigenetics. 2010 Aug 16;5(6):491-8. doi: 10.4161/epi.5.6.12431.
Hao X, Luo H, Krawczyk M, Wei W, Wang W, Wang J, et al. DNA methylation markers for diagnosis and prognosis of common cancers. Proc Natl Acad Sci U S A. 2017 Jul 11;114(28):7414-7419. doi: 10.1073/pnas.1703577114.
Munemitsu S, Souza B, Müller O, Albert I, Rubinfeld B, Polakis P. The APC gene product associates with microtubules in vivo and promotes their assembly in vitro. Cancer Res. 1994 Jul 15;54(14):3676-81.
Barth AI, Pollack AL, Altschuler Y, Mostov KE, Nelson WJ. NH2-terminal deletion of beta-catenin results in stable colocalization of mutant beta-catenin with adenomatous polyposis coli protein and altered MDCK cell adhesion. J Cell Biol. 1997 Feb 10;136(3):693-706. doi: 10.1083/jcb.136.3.693.
Munemitsu S, Souza B, Müller O, Albert I, Rubinfeld B, Polakis P. The APC gene product associates with microtubules in vivo and promotes their assembly in vitro. Cancer Res. 1994 Jul 15;54(14):3676-81.
Neufeld KL, White RL. Nuclear and cytoplasmic localizations of the adenomatous polyposis coli protein. Proc Natl Acad Sci U S A. 1997 Apr 1;94(7):3034-9. doi: 10.1073/pnas.94.7.3034.
Henderson BR. Nuclear-cytoplasmic shuttling of APC regulates beta-catenin subcellular localization and turnover. Nat Cell Biol. 2000 Sep;2(9):653-60. doi: 10.1038/35023605.
Fearnhead NS, Britton MP, Bodmer WF. The ABC of APC. Hum Mol Genet. 2001 Apr;10(7):721-33. doi: 10.1093/hmg/10.7.721.
Goss KH, Groden J. Biology of the adenomatous polyposis coli tumor suppressor. J Clin Oncol. 2000 May;18(9):1967-79. doi: 10.1200/JCO.2000.18.9.1967.
Ishidate T, Matsumine A, Toyoshima K, Akiyama T. The APC-hDLG complex negatively regulates cell cycle progression from the G0/G1 to S phase. Oncogene. 2000 Jan 20;19(3):365-72. doi: 10.1038/sj.onc.1203309.
Nakamura M, Zhou XZ, Lu KP. Critical role for the EB1 and APC interaction in the regulation of microtubule polymerization. Curr Biol. 2001 Jul 10;11(13):1062-7. doi: 10.1016/s0960-9822(01)00297-4.
Behrens J, von Kries JP, Kühl M, Bruhn L, Wedlich D, Grosschedl R, Birchmeier W. Functional interaction of beta-catenin with the transcription factor LEF-1. Nature. 1996 Aug 15;382(6592):638-42. doi: 10.1038/382638a0.
Morin, P. J., Sparks, A. B., Korinek, V., Barker, N., et al. Clevers, H., Vogelstein, B., & Kinzler, K. W. Activation of β-catenin-Tcf signaling in colon cancer by mutations in β-catenin or APC. Science, 275.5307 (1997):1787-1790.
Korinek V, Barker N, Morin PJ, van Wichen D, de Weger R, Kinzler KW, et al. Constitutive transcriptional activation by a beta-catenin-Tcf complex in APC-/- colon carcinoma. Science. 1997 Mar 21;275(5307):1784-7. doi: 10.1126/science.275.5307.1784.
He TC, Sparks AB, Rago C, Hermeking H, Zawel L, da Costa LT, et al. Identification of c-MYC as a target of the APC pathway. Science. 1998 Sep 4;281(5382):1509-12. doi: 10.1126/science.281.5382.1509.
Shtutman M, Zhurinsky J, Simcha I, Albanese C, D'Amico M, Pestell R, et al. The cyclin D1 gene is a target of the beta-catenin/LEF-1 pathway. Proc Natl Acad Sci U S A. 1999 May 11;96(10):5522-7. doi: 10.1073/pnas.96.10.5522.
Mann B, Gelos M, Siedow A, Hanski ML, Gratchev A, Ilyas M, et al. Target genes of beta-catenin-T cell-factor/lymphoid-enhancer-factor signaling in human colorectal carcinomas. Proc Natl Acad Sci U S A. 1999 Feb 16;96(4):1603-8. doi: 10.1073/pnas.96.4.1603.
Jaiswal AS, Narayan S. A novel function of adenomatous polyposis coli (APC) in regulating DNA repair. Cancer Lett. 2008 Nov 28;271(2):272-80. doi: 10.1016/j.canlet.2008.06.024.
Miki Y, Swensen J, Shattuck-Eidens D, Futreal PA, Harshman K, Tavtigian S, et al. A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science. 1994 Oct 7;266(5182):66-71. doi: 10.1126/science.7545954.
Friedman LS, Ostermeyer EA, Szabo CI, Dowd P, Lynch ED, Rowell SE, King MC. Confirmation of BRCA1 by analysis of germline mutations linked to breast and ovarian cancer in ten families. Nat Genet. 1994 Dec;8(4):399-404. doi: 10.1038/ng1294-399.
Catteau A, Morris JR. BRCA1 methylation: a significant role in tumour development? Semin Cancer Biol. 2002 Oct;12(5):359-371. doi: 10.1016/s1044-579x(02)00056-1. Erratum in: Semin Cancer Biol. 2003 Feb;13(1):91.
Scully R, Chen J, Ochs RL, Keegan K, Hoekstra M, Feunteun J, Livingston DM. Dynamic changes of BRCA1 subnuclear location and phosphorylation state are initiated by DNA damage. Cell. 1997 Aug 8;90(3):425-35. doi: 10.1016/s0092-8674(00)80503-6.
Hartwell LH, Weinert TA. Checkpoints: controls that ensure the order of cell cycle events. Science. 1989 Nov 3;246(4930):629-34. doi: 10.1126/science.2683079.
Fabbro M, Savage K, Hobson K, Deans AJ, Powell SN, McArthur GA, Khanna KK. BRCA1-BARD1 complexes are required for p53Ser-15 phosphorylation and a G1/S arrest following ionizing radiation-induced DNA damage. J Biol Chem. 2004 Jul 23;279(30):31251-8. doi: 10.1074/jbc.M405372200.
Bernstein C, Bernstein H, Payne CM, Garewal H. DNA repair/pro-apoptotic dual-role proteins in five major DNA repair pathways: fail-safe protection against carcinogenesis. Mutat Res. 2002 Jun;511(2):145-78. doi: 10.1016/s1383-5742(02)00009-1.
Wu J, Lu LY, Yu X. The role of BRCA1 in DNA damage response. Protein Cell. 2010 Feb;1(2):117-23. doi: 10.1007/s13238-010-0010-5.
Dobrovic A, Simpfendorfer D. Methylation of the BRCA1 gene in sporadic breast cancer. Cancer Res. 1997 Aug 15;57(16):3347-50.
Rice JC, Ozcelik H, Maxeiner P, Andrulis I, Futscher BW. Methylation of the BRCA1 promoter is associated with decreased BRCA1 mRNA levels in clinical breast cancer specimens. Carcinogenesis. 2000 Sep;21(9):1761-5. doi: 10.1093/carcin/21.9.1761.
Morizono A, Tanabe M, Ikemura M, Sasaki T, Ushiku T, Seto Y. Loss of BRCA1 expression and morphological features associated with BRCA1 promoter methylation status in triple-negative breast cancer. J Hum Genet. 2021 Aug;66(8):785-793. doi: 10.1038/s10038-021-00911-3.
S SK, Swamy SN, Premalatha CS, Pallavi VR, Gawari R. Aberrant Promoter Hypermethylation of RASSF1a and BRCA1 in Circulating Cell-Free Tumor DNA Serves as a Biomarker of Ovarian Carcinoma. Asian Pac J Cancer Prev. 2019 Oct 1;20(10):3001-3005. doi: 10.31557/APJCP.2019.20.10.3001.
Kalachand RD, Stordal B, Madden S, Chandler B, Cunningham J, Goode EL, et al. BRCA1 Promoter Methylation and Clinical Outcomes in Ovarian Cancer: An Individual Patient Data Meta-Analysis. J Natl Cancer Inst. 2020 Dec 14;112(12):1190-1203. doi: 10.1093/jnci/djaa070.
Tabano S, Azzollini J, Pesenti C, Lovati S, Costanza J, Fontana L, et al. Analysis of BRCA1 and RAD51C Promoter Methylation in Italian Families at High-Risk of Breast and Ovarian Cancer. Cancers (Basel). 2020 Apr 8;12(4):910. doi: 10.3390/cancers12040910.
Baylin SB. DNA methylation and gene silencing in cancer. Nat Clin Pract Oncol. 2005 Dec;2 Suppl 1:S4-11. doi: 10.1038/ncponc0354.
Jones PA, Baylin SB. The fundamental role of epigenetic events in cancer. Nat Rev Genet. 2002 Jun;3(6):415-28. doi: 10.1038/nrg816.
Kamiyama H, Noda H, Takata O, Suzuki K, Kawamura Y, Konishi F. Promoter hypermethylation of tumor-related genes in peritoneal lavage and the prognosis of patients with colorectal cancer. J Surg Oncol. 2009 Jul 1;100(1):69-74. doi: 10.1002/jso.21291.
Lee BB, Lee EJ, Jung EH, Chun HK, Chang DK, Song SY, et al. Aberrant methylation of APC, MGMT, RASSF2A, and Wif-1 genes in plasma as a biomarker for early detection of colorectal cancer. Clin Cancer Res. 2009 Oct 1;15(19):6185-91. doi: 10.1158/1078-0432.CCR-09-0111.
Matthaios D, Balgkouranidou I, Karayiannakis A, Bolanaki H, Xenidis N, Amarantidis K, et al. Methylation status of the APC and RASSF1A promoter in cell-free circulating DNA and its prognostic role in patients with colorectal cancer. Oncol Lett. 2016 Jul;12(1):748-756. doi: 10.3892/ol.2016.4649.
Chen J, Röcken C, Lofton-Day C, Schulz HU, Müller O, Kutzner N, et al. Molecular analysis of APC promoter methylation and protein expression in colorectal cancer metastasis. Carcinogenesis. 2005 Jan;26(1):37-43. doi: 10.1093/carcin/bgh280.
Van der Auwera I, Van Laere SJ, Van den Bosch SM, Van den Eynden GG, Trinh BX, van Dam PA, et al. Aberrant methylation of the Adenomatous Polyposis Coli (APC) gene promoter is associated with the inflammatory breast cancer phenotype. Br J Cancer. 2008 Nov 18;99(10):1735-42. doi: 10.1038/sj.bjc.6604705.
Tsuchiya T, Tamura G, Sato K, Endoh Y, Sakata K, Jin Z, et al. Distinct methylation patterns of two APC gene promoters in normal and cancerous gastric epithelia. Oncogene. 2000 Jul 27;19(32):3642-6. doi: 10.1038/sj.onc.1203704.
Brabender J, Usadel H, Danenberg KD, Metzger R, Schneider PM, Lord RV, et al. Adenomatous polyposis coli gene promoter hypermethylation in non-small cell lung cancer is associated with survival. Oncogene. 2001 Jun 14;20(27):3528-32. doi: 10.1038/sj.onc.1204455.
Ignatov A, Bischoff J, Ignatov T, Schwarzenau C, Krebs T, Kuester D, et al. APC promoter hypermethylation is an early event in endometrial tumorigenesis. Cancer Sci. 2010 Feb;101(2):321-7. doi: 10.1111/j.1349-7006.2009.01397.x.
Zysman M, Saka A, Millar A, Knight J, Chapman W, Bapat B. Methylation of adenomatous polyposis coli in endometrial cancer occurs more frequently in tumors with microsatellite instability phenotype. Cancer Res. 2002 Jul 1;62(13):3663-6.
Ghazanfari T, Asaadi Tehrani G, Maziri P. The Relationship between the Methylation of Promoter Regions of Tumor Suppressor Genes PTEN and APC with Endometrial Cancer. Asian Pac J Cancer Prev. 2019 Aug 1;20(8):2259-2265. doi: 10.31557/APJCP.2019.20.8.2259.
Zhang L, Long X. Association of BRCA1 promoter methylation with sporadic breast cancers: Evidence from 40 studies. Sci Rep. 2015 Dec 8;5:17869. doi: 10.1038/srep17869.
Ignatov T, Poehlmann A, Ignatov A, Schinlauer A, Costa SD, Roessner A, et al. BRCA1 promoter methylation is a marker of better response to anthracycline-based therapy in sporadic TNBC. Breast Cancer Res Treat. 2013 Sep;141(2):205-12. doi: 10.1007/s10549-013-2693-9.
Buyru N, Altinisik J, Ozdemir F, Demokan S, Dalay N. Methylation profiles in breast cancer. Cancer Invest. 2009 Mar;27(3):307-12. doi: 10.1080/07357900802350814.
Hsu NC, Huang YF, Yokoyama KK, Chu PY, Chen FM, Hou MF. Methylation of BRCA1 promoter region is associated with unfavorable prognosis in women with early-stage breast cancer. PLoS One. 2013;8(2):e56256. doi: 10.1371/journal.pone.0056256.
Kalachand RD, Stordal B, Madden S, Chandler B, Cunningham J, Goode EL, et al. BRCA1 Promoter Methylation and Clinical Outcomes in Ovarian Cancer: An Individual Patient Data Meta-Analysis. J Natl Cancer Inst. 2020 Dec 14;112(12):1190-1203. doi: 10.1093/jnci/djaa070. PMID: 32413141; PMCID: PMC7735770.
Baldwin RL, Nemeth E, Tran H, Shvartsman H, Cass I, Narod S, Karlan BY. BRCA1 promoter region hypermethylation in ovarian carcinoma: a population-based study. Cancer Res. 2000 Oct 1;60(19):5329-33.
Esteller M, Silva JM, Dominguez G, Bonilla F, Matias-Guiu X, Lerma E, et alPromoter hypermethylation and BRCA1 inactivation in sporadic breast and ovarian tumors. J Natl Cancer Inst. 2000 Apr 5;92(7):564-9. doi: 10.1093/jnci/92.7.564.
Dhillon VS, Aslam M, Husain SA. The contribution of genetic and epigenetic changes in granulosa cell tumors of ovarian origin. Clin Cancer Res. 2004 Aug 15;10(16):5537-45. doi: 10.1158/1078-0432.CCR-04-0228.
Wiley A, Katsaros D, Chen H, Rigault de la Longrais IA, Beeghly A, Puopolo M, et al. Aberrant promoter methylation of multiple genes in malignant ovarian tumors and in ovarian tumors with low malignant potential. Cancer. 2006 Jul 15;107(2):299-308. doi: 10.1002/cncr.21992.
Mancini DN, Rodenhiser DI, Ainsworth PJ, O'Malley FP, Singh SM, Xing W, et al. CpG methylation within the 5' regulatory region of the BRCA1 gene is tumor specific and includes a putative CREB binding site. Oncogene. 1998 Mar 5;16(9):1161-9. doi: 10.1038/sj.onc.1201630.
An J, Wei Q, Liu Z, Lu KH, Cheng X, Mills GB, Wang LE. Messenger RNA expression and methylation of candidate tumor-suppressor genes and risk of ovarian cancer-a case-control analysis. Int J Mol Epidemiol Genet. 2010;1(1):1-10.
Copyright (c) 2021 Author (s). Published by Siddharth Health Research and Social Welfare Society

This work is licensed under a Creative Commons Attribution 4.0 International License.


 OAI - Open Archives Initiative
OAI - Open Archives Initiative


