Autologous Whole Blood Injection For COVID-19 Can Reduce Cytokine Storm and Severity of Illness
Abstract
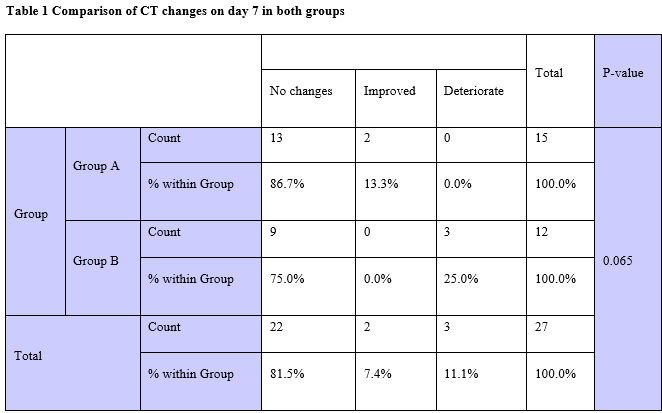
Autologous whole blood injection is used for various indications. It has an immunomodulatory action on the immune system. A randomized controlled two-arm study was conducted to determine IL-6 levels, CT changes and mortality among adult COVID-19 patients. The trial included 30 patients divided into two groups. The interventional group received 2 doses of 2.5 ml of autologous whole blood injection spaced 2 days apart. There was a statistically significant reduction in IL-6 levels on day 6 in the group receiving treatment. CT score improved in patients who received treatment. No cases of mortality were reported in the treatment group. Autologous whole blood injection can be used as a simple, low-cost adjuvant in the treatment of adult COVID-19 patients, regardless of disease severity.
Downloads
References
WHO Working Group on the Clinical Characterisation and Management of COVID-19 infection. A minimal common outcome measure set for COVID-19 clinical research. Lancet Infect Dis. 2020 Aug;20(8):e192-e197. doi: 10.1016/S1473-3099(20)30483-7.
Hojyo S, Uchida M, Tanaka K, Hasebe R, Tanaka Y, Murakami M, et al. How COVID-19 induces cytokine storm with high mortality. Inflamm Regen. 2020 Oct 1;40:37. doi: 10.1186/s41232-020-00146-3.
Gupta S, Leaf DE. Tocilizumab in COVID-19: some clarity amid controversy. Lancet. 2021 May 1;397(10285):1599-1601. doi: 10.1016/S0140-6736(21)00712-1.
Chen LYC, Hoiland RL, Stukas S, Wellington CL, Sekhon MS. Confronting the controversy: interleukin-6 and the COVID-19 cytokine storm syndrome. Eur Respir J. 2020 Oct 1;56(4):2003006. doi: 10.1183/13993003.03006-2020.
Diehl S, Rincón M. The two faces of IL-6 on Th1/Th2 differentiation. Mol Immunol. 2002 Dec;39(9):531-6. doi: 10.1016/s0161-5890(02)00210-9.
Oomen-Welke K, Huber R. Intramuscular autologous blood therapy - a systematic review of controlled trials. BMC Complement Altern Med. 2019 Sep 5;19(1):248. doi: 10.1186/s12906-019-2643-0.
Olwin JH, Ratajczak HV, House RV. Successful treatment of herpetic infections by autohemotherapy. J Altern Complement Med. 1997 Summer;3(2):155-8. doi: 10.1089/acm.1997.3.155.
Gil-Etayo FJ, Suàrez-Fernández P, Cabrera-Marante O, Arroyo D, Garcinuño S, Naranjo L, Pleguezuelo DE, Allende LM, Mancebo E, Lalueza A, Díaz-Simón R, Paz-Artal E, Serrano A. T-Helper Cell Subset Response Is a Determining Factor in COVID-19 Progression. Front Cell Infect Microbiol. 2021 Feb 26;11:624483. doi: 10.3389/fcimb.2021.624483.
Hakimi J, Azizi A, Ausar SF, Todryk SM, Rahman N, Brookes RH. An adjuvant-modulated vaccine response in human whole blood. Hum Vaccin Immunother. 2017 Sep 2;13(9):2130-2134. doi: 10.1080/21645515.2017.1337616.
Sheikhi A, Azarbeig M, Karimi H. Autohemotherapy in chronic urticaria: what could be the autoreactive factors and curative mechanisms? Ann Dermatol. 2014 Aug;26(4):526-7. doi: 10.5021/ad.2014.26.4.526.
Gustine JN, Jones D. Immunopathology of Hyperinflammation in COVID-19. Am J Pathol. 2021 Jan;191(1):4-17. doi: 10.1016/j.ajpath.2020.08.009.
Copyright (c) 2021 Author (s). Published by Siddharth Health Research and Social Welfare Society

This work is licensed under a Creative Commons Attribution 4.0 International License.


 OAI - Open Archives Initiative
OAI - Open Archives Initiative


