Study of knowledge, perception, and practice of patients regarding fasting requirements for blood glucose testing
Abstract
Background: Patient preparation is one of the least standardized parts of the preanalytic phase of testing. Fasting blood glucose requires fasting for 8-12 hours as per various guidelines and also has several other requirements. Lack of communication, understanding, or compliance regarding hours-of-fasting, water-intake, avoidance of caloric snack/beverage, the sudden change in smoking, exercise, alcohol, medication, etc. introduces preanalytic errors.
Method: To evaluate awareness, understanding, and compliance with fasting requirements, a face-to-face survey was done on outpatients in a Government Hospital in Pali, Rajasthan, India. Relatively more educated internet users were surveyed as controls through an online SurveyMonkey tool. Information collected included demographics, perception of above mentioned preanalytic factors related to fasting, and compliance.
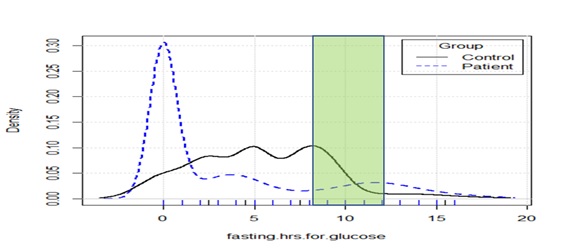
Results: 98 patients and 187 controls participated in the study. Perception about fasting requirements ranged from 0-17 hours. 71% of patients and 35% of controls perceived that nobody explained to them the duration or nature of fasting. The different sources of information had been used in different proportions by patients and controls. For imparting understanding and compliance about duration, and other requirements of fasting, the instruction was usually incomplete but still much more effective (p-value=0.000002) than formal education level (p-value=0.024). Financial status had a weak negative association with awareness but was not significant.
Conclusion: 71% of patients and 35% of controls did not receive instructions for fasting. 40% of those instructed showed better compliance, but awareness was incomplete. The instruction was more effective than formal education in improving awareness and compliance. Improved awareness was strongly associated with receiving instruction and weakly associated with formal education but financial status showed only a weak negative association.
Downloads
References
Lippi G, Banfi G, Church S, Cornes M, De Carli G, Grankvist K, et al. Preanalytical quality improvement. In pursuit of harmony, on behalf of European Federation for Clinical Chemistry and Laboratory Medicine (EFLM) Working group for Preanalytical Phase (WG-PRE). Clin Chem Lab Med. 2015;53(3):357-370. doi: 10.1515/cclm-2014-1051.
Plebani M, Lippi G. Closing the brain-to-brain loop in laboratory testing. Clin Chem Lab Med. 2011;49(7):1131–1133. doi: 10.1515/CCLM.2011.617.
Salinas M, Flores E, Puche CM, Lopez-Garrigos M, Leiva-Salinas C. Recommendations for the patient preparation for laboratory tests in primary care in Spain: A redconlab study. Clinica Chimica Acta. 2019;493:S705. doi: 10.1016/j.cca.2019.03.1560.
Simundic AM, Cornes M, Grankvist K, Lippi G, Nybo M. Standardization of collection requirements for fasting samples: for the Working Group on Preanalytical Phase (WG-PA) of the European Federation of Clinical Chemistry and Laboratory Medicine (EFLM). Clinica Chimica Acta. 2014;432:33-37. doi: 10.1016/j.cca.2013.11.008
Lima-Oliveira G, Salvagno GL, Lippi G, Gelati M, Montagnana M, Danese E, et al. Influence of a regular, standardized meal on clinical chemistry analytes. Ann Lab Med. 2012;32(4):250-256. doi: 10.3343/alm.2012.32.4.250.
Guidi GC, Simundic AM, Salvagno GL, Aquino JL, Lima-Oliveira G. To avoid fasting time, more risk than benefits. Clin Chem Lab Med (CCLM). 2015;53(10), e261–e264. doi: 10.1515/cclm-2014-1013.
Lippi G, Lima-Oliveira G, Salvagno GL, Montagnana M, Gelati M, Picheth G, et al. Influence of a light meal on routine haematological tests. Blood Transfus. 2010;8(2):94-99. doi: 10.2450/2009.0142-09.
Moebus S, Göres L, Lösch C, Jöckel KH. Impact of time since last caloric intake on blood glucose levels. Europe J Epidemiol. 2011;26(9), 719–728. doi: 10.1007/s10654-011-9608-z.
Nybo M, Grinsted P, Jørgensen PE. Blood Sampling: Is Fasting Properly Defined? Clin Chem. 2005;51(8):1563-1564. doi: 10.1373/clinchem.2005.051789.
Kackov S, Simundic AM., Gatti-Drnic A. Are patients well informed about the fasting requirements for laboratory blood testing? Biochemia Medica. 2013;23(3):326-331. doi: 10.11613/bm.2013.040.
Nikolac N, Simundic AM, Kackov S, Serdar T, Dorotic A, Fumic K, et al. The quality and scope of information provided by medical laboratories to patients before laboratory testing: Survey of the Working Group for Patient Preparation of the Croatian Society of Medical Biochemistry and Laboratory Medicine. Clinica Chimica Acta. 2015;450:104-109. doi: 10.1016/j.cca.2015.08.001.
POCT13: Glucose Monitoring Without Lab Support - CLSI. (n.d.). Clinical & Laboratory Standards Institute. Retrieved December 25, 2019, Available from https://clsi.org/standards/products/point-of-care-testing/documents/poct13/.
Simundic AM, Bölenius K, Cadamuro J, Church S, Cornes MP, van Dongen-Lases EC, et al. Working Group for Preanalytical Phase (WG-PRE), of the European Federation of Clinical Chemistry and Laboratory Medicine (EFLM) and Latin American Working Group for Preanalytical Phase (WG-PRE-LATAM) of the Latin America Confederation of Clinical Biochemistry (COLABIOCLI) Joint EFLM-COLABIOCLI Recommendation for venous blood sampling. Clin Chem Lab Med. 2018;56(12):2015-2038. doi: 10.1515/cclm-2018-0602.
World Health Organization. (1999). Definition, diagnosis and classification of diabetes mellitus and its complications: Report of a WHO consultation. Part 1, Diagnosis and classification of diabetes mellitus. World Health Organization. Available from https://apps.who.int/iris/bitstream/handle/10665/66040/WHO_NCD_NCS_99.2.pdf.
Citing SurveyMonkey. (n.d.). Retrieved September 25, 2019, Available from https://help.surveymonkey.com/articles/en_US/kb/May-I-reference-SurveyMonkey-in-a-paper-or-thesis.
Dalvi TM, Khairnar MR, Kalghatgi SR. An Update of B.G. Prasad and Kuppuswamy Socio-Economic Status Classification Scale for Indian Population. Indian J Pediatr. 2020;87(7):567-568. doi: 10.1007/s12098-020-03200-7.
Hallworth MJ. The ‘70% claim’: What is the evidence base? Ann Clin Biochem. 2017;48(6):487-488. doi: 10.1258/acb.2011.011177.
Salinas M, López-Garrigós M, Flores E, Leiva-Salinas C. Current Practice and Regional Variability in Recommendations for Patient Preparation for Laboratory Testing in Primary Care. Lab Med. 2020;51(3):e32-e37. doi: 10.1093/labmed/lmz092.
Joshi SR, Parikh RM. India; the diabetes capital of the world: Now heading towards hypertension. J Assoc Physic India. 2007;55(Y):323.
Tandon N, Anjana RM, Mohan V, Kaur T, Afshin A, Ong K, et al. The increasing burden of diabetes and variations among the states of India: the Global Burden of Disease Study 1990–2016. Lancet Global Health. 2018;6(12):e1352-e1362. doi: 10.1016/S2214-109X(18)30387-5.
Altuntaş Y. Postprandial Reactive Hypoglycemia. Şişli Etfal Hastanesi Tıp Bülteni 2019;53(3):215-220. doi: 10.14744/SEMB.2019.59455.
Sørensen M, Johansen OE. Idiopathic reactive hypoglycaemia–prevalence and effect of fibre on glucose excursions. Scandinavian J Clin Lab Investig. 2010;70(6):385-391. doi: 10.3109/00365513.2010.491869.
Lippi G, Simundic AM, & European Federation for Clinical Chemistry and Laboratory Medicine (EFLM) Working Group for Preanalytical Phase (WG-PRE). The EFLM strategy for harmonization of the preanalytical phase. Clin Chem Lab Med. 2018;56(10):1660-1666. doi: 10.1515/cclm-2017-0277.
Kljakovic M. Patients and tests: A study into patient understanding of blood tests ordered by their doctor. Australian Family Physician. 2012;41(4):241.
Pant V, Gautam K, Pradhan S. Fasting Blood Glucose Test in Nepal-Time for a Harmonized Definition. J Nepal Health Res Council. 2019;17(2):267-268. doi: 10.33314/jnhrc.v0i0.1810.
New World Bank country classifications by income level: 2020-2021. (n.d.). Retrieved July 17, 2020, Available from https://blogs.worldbank.org/opendata/new-world-bank-country-classifications-income-level-2020-2021
Doe, J. (2012, May 21). Eurobarometer Qualitative Study on patient involvement in healthcare [Text]. EUROPEAN INNOVATION PARTNERSHIP - European Commission. Available from https://ec.europa.eu/eip/ageing/library/eurobarometer-qualitative-study-patient-involvement-healthcare_en.
Copyright (c) 2021 Author (s). Published by Siddharth Health Research and Social Welfare Society

This work is licensed under a Creative Commons Attribution 4.0 International License.


 OAI - Open Archives Initiative
OAI - Open Archives Initiative


