Assessment of levels of Vitamin D and Leptin in comparison of BMI among medical students
Abstract
Introduction: Vitamin D is one of fat-soluble vitamin that plays an important role in the absorption of calcium and phosphate. Deficiency of Vitamin D is unrecognized in many parts of the world. Leptin is a hormone which is derived from adipose tissue. Studies have shown that vitamin D has a negative and powerful control on leptin secretion by vitamin D by acting on the adipose tissue.
Aim and Objectives: The study was done to study the relationship between Vitamin D and Leptin based on Body mass index among the medical students.
Materials and methods: Vitamin D Leptin and Body mass index were the parameters measured in the study group. Individuals with an age group of 19-23 years of both sexes were included in the study. Individuals above the age of 23 years, those with renal and liver disorders, individuals with hormonal disorders, individuals on vitamin D supplementation were excluded in the study. Vitamin D was measured by Enzyme-Linked Immunosorbent Assay (ELISA) method. Leptin was measured by Enzyme-Linked Immunosorbent Assay (ELISA) method. BMI is calculated by the formula weight in kilograms divided by height in metre square.
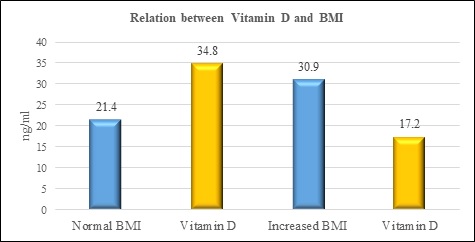
Results: The results have shown that there is a decrease in vitamin D levels with increasing BMI. (pvalue≤0.001). furthermore, there is an increase in leptin levels with an increase in BMI. (pvalue≤0.001).
Conclusion: The study has put forth a suggestion that leptin and vitamin D has a causal relationship between them based on Body Mass index. Adequate vitamin D levels will maximize the effect of maintaining normal leptin levels as high levels of leptin could contribute to obesity-related disorders.
Downloads
References
Holick MF. Vitamin D: the underappreciated D-lightful hormone that is important for skeletal and cellular health. Curr Opin Endocrinol Diabetes.2002;9(1):87-98. doi: https://doi.org/10.1097/00060793-200202000-00011.
Tangpricha V, Koutkia P, Rieke SM, Chen TC, Perez AA, Holick MF. Fortification of orange juice with vitamin D: a novel approach to enhance vitamin D nutritional health. Am J Clin Nutr. 2003;77(6):1478-1483. doi: https://doi.org/10.1093/ajcn/77.6.1478.
Webb AR, deCosta BR, Holick MF. Sunlight regulates the cutaneous production of vitamin D3 by causing its photodegradation. J Clin Endocrinol Metab. 1989;68(5):882-887. doi: https://doi.org/10.1210/jcem-68-5-882.
Clemens TL, Adams JS, Henderson SL, Holick MF. Increased skin pigment reduces the capacity of skin to synthesise vitamin D3. Lancet. 1982;1(8263):74-76. doi: https://doi.org/10.1016/s0140-6736(82)90214-8.
Matsuoka LY, Ide L, Wortsman J, MacLaughlin JA, Holick MF. Sunscreens supress cutaneous vitamin D3 synthesis. J Clin Endocrinol Metab. 1987;64(6):1165-1168. doi: https://doi.org/10.1210/jcem-64-6-1165.
Bikle DD. Vitamin D: role in skin and hair. In: Feldman D, ed. Vitamin D. Vol 1. 2nd ed. Elsevier Academic Press; 2005:609-630. San Diego, California.
Hosomi J, Hosoi J, Abe E, Suda T, Kuroki T. Regulation of terminal differentiation of cultured mouse epidermal cells by 1 alpha,25-dihydroxyvitamin D3. Endocrinol. 1983;113(6):1950-1957. doi: https://doi.org/10.1210/endo-113-6-1950.
Smith EL, Walworth NC, Holick MF. Effect of 1α,25-dihydroxyvitamin D3 on the morphologic and biochemical differentiation of cultured human epidermal keratinocytes grown in serum-free conditions. J Invest Dermatol. 1986;86(6):709-714. doi: https://doi.org/10.1111/1523-1747.ep12276343.
Heaney RP, Dowell MS, Hale CA, Bendich A. Calcium absorption varies within the reference range for serum 25-hydroxyvitamin D. J Am Coll Nutr. 2003;22(2):142-146. doi: https://doi.org/10.1080/07315724.2003.10719287.
Binkley N, Krueger D, Cowgill CS, Plum L, Lake E, Hansen KE, et al. Assay variation confounds the diagnosis of hypovitaminosis D: a call for standardization. J Clin Endocrinol. Metab. 2004; 89(7):3152-3157. doi: https://doi.org/10.1210/jc.2003-031979.
Lips P, Chapuy MC, Dawson-Hughes B, Pols HA, Holick MF. An international comparision of serum 25-hydroxyvitamin D measurements. Osteoporos Int. 1999;9(5):394-397. doi: https://doi.org/10.1007/s001980050162.
Maetani M, Maskarinec G, Franke AA, Cooney RV. Association of leptin, 25-hydroxyvitamin D, and parathyroid hormone in women. Nutri Can. 2009;61(2):225-231. doi: https://dx.doi.org/10.1080%2F01635580802455149.
Schwartz GG, Skinner HG. Vitamin D status and cancer: new insights. Curr Opin Clin Nutr Metabolic Care. 2007;10(1):6-11. doi: https://doi.org/10.1097/mco.0b013e328011aa60.
Spina CS, Tangpricha V, Uskokovic M, Adorinic L, Maehr H, et al. Vitamin D and cancer. Anticancer Res. 2006; 26(4A):2515-2524.
Wu K, Feskanich D, Fuchs CS, Willett WC, Hollis BW, et al. A nested case control study of plasma 25-hydroxyvitamin D concentrations and risk of colorectal cancer. J Natl Cancer Inst. 2007;99(14):1120-1129. doi: https://doi.org/10.1093/jnci/djm038.
Zittermann A. Vitamin D and disease prevention with special reference to cardiovascular disease. Prog Biophys Mol Biol. 2006;92(1):39-48. doi: https://doi.org/10.1016/j.pbiomolbio.2006.02.001.
Autier P, Gandini S. Vitamin D supplementation and total mortality: a meta-analysis of randomized controlled trials. Arch Intern Med. 2007;167(16):1730-1737.
Dong F, Ren J. Fitness or fatness—the debate continues for the role of leptin in obesity-associated heart dysfunction. Curr Diabetes Rev. 2007;3(3):159-164. doi: https://doi.org/10.2174/157339907781368959.
Calle EE, Thun MJ, Petrelli JM, Rodriguez C, Heath CW. Body-mass index and mortality in a prospective cohort of U.S. adults. New Engl J Med. 1999;341(15):1097-1105. doi: https://doi.org/10.1056/nejm199910073411501.
Moghaddam AA, Woodward M, Huxley R. Obesity and risk of colorectal cancer: a meta-analysis of 31 studies with 70,000 events. Cancer Epidem Biomarkers Prev. 2007;16(12):2533-2547. doi: https://doi.org/10.1158/1055-9965.epi-07-0708.
WHO Mean Body Index (BMI) World Health Organization. Available at https://www.who.int/gho/ncd/risk_factors/bmi_text/en/.
Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371(9612):569-578. doi: https://doi.org/10.1016/s0140-6736(08)60269-x.
Buschemeyer WC III, Freedland SJ. Obesity and prostate cancer: epidemiology and clinical implications. Eur Urol. 2007;52(2):331-343. doi: https://doi.org/10.1016/j.eururo.2007.04.069.
Matsunuma A, Kawane T, Maeda T, Hamada S, Horiuchi N. Leptin corrects increased gene expression of renal 25-hydroxyvitamin D3–1-hydroxylase and-24-hydroxylase in leptin-deficient,ob/ob mice. Endocrinol. 2004;145(3):1367-1375. doi: https://doi.org/10.1210/en.2003-1010.
Glauber HS, Vollmer WM, Nevitt MC, Ensrud KE, Orwoll ES. Body weight versus body fat distribution, adiposity, and frame size as predictors of bone density. J Clin Endocrinol Metab. 1995;80(4):1118-1123. doi: https://doi.org/10.1210/jcem.80.4.7714079.
Menendez C, Lage M, Peino R, Baldelli R, Concheiro P, et al.: Retinoic acid and vitamin D (3) powerfully inhibit in vitro leptin secretion by human adipose tissue. J Endocrinol. 2001;170(2):425-431. doi: https://doi.org/10.1677/joe.0.1700425.
Park K. Text book of preventive and social Medicine 19th ed, page 335.
Konradsen S, Ag H, Lindberg F, Hexeberg S, Jorde R. Serum 1,25-dihydroxy vitamin D is inversely associated with body mass index. Eur J Nutr. 2008;47(2):87-91. doi: https://doi.org/10.1007/s00394-008-0700-4.
Li J, Byrne ME, Chang E, Jiang Y, Donkin SS, Buhman KK et al. 1alpha,25-DihydroxyvitaminD hydroxylase in adipocytes.J Steroid Biochem Mol Biol. 2008;112(1-3):122-126. doi: https://doi.org/10.1016/j.jsbmb.2008.09.006.
Trayhurn P, Wood IS. Adipokines: inflammation and the pleiotropic role of white adipose tissue. Brit J Nutr. 2004; 92(3):347-355. doi: https://doi.org/10.1079/bjn20041213.
Pelleymounter MA, Cullen MJ, Baker MB, Hecht R, Winters D, Boone T, et al. Effects of the obese gene product on body weight regulation in ob/ob mice. Sci.1995,269(5223):540-543. doi: https://doi.org/10.1126/science.7624776.
Lönnqvist F, Arner P, Nordfors L, Schalling M. Overexpression of the obese (ob) gene in adipose tissue of human obese subjects. Nat Med. 1995;1(9):950-953. doi: https://doi.org/10.1038/nm0995-950.
De Vos P, Saladin R, Auwerx J, Staels B. Induction of ob gene expression by corticosteroids is accompanied by body weight loss and reduced food intake. J Biol Chem. 1995;270(27):15958-15961. doi: https://doi.org/10.1074/jbc.270.27.15958.


 OAI - Open Archives Initiative
OAI - Open Archives Initiative


