A prospective study comparing induction chemotherapy followed by chemoradiation versus chemoradiation alone in stage III non-small cell lung cancer
Abstract
Background: Although concurrent chemoradiation (CCRT) is the standard of care for stage III non-small cell lung cancer(NSCLC), the five years overall (OS) survival is very poor. Most of the patients developed distant metastasis later which can be improved by induction chemotherapy.
Aims: This study was designed to observe the difference in epidemiology, acute toxicities, overall responses [complete response (CR)+partial response (PR)] after treatment completion, disease-free survival (DFS) and progression-free survival (PFS) at the end of the study.
Settings and Design: This was a prospective, interventional, randomized hospital-based study.
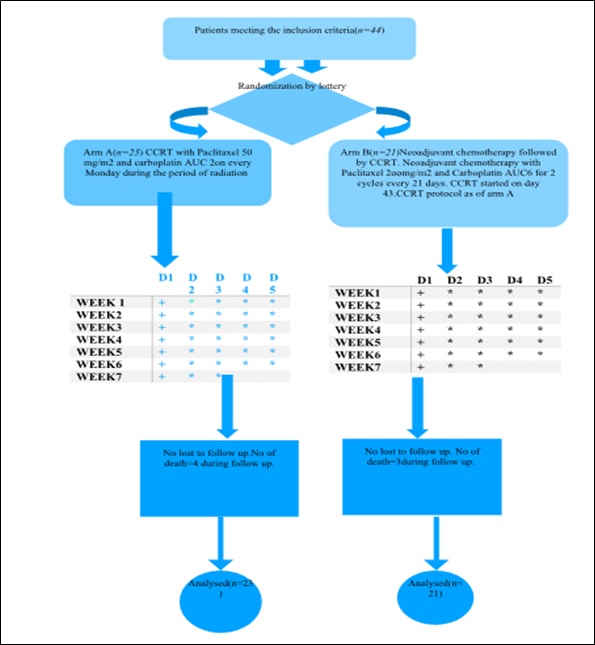
Methods and Material: Eligible patients were randomized into arm A (CCRT with weekly paclitaxel(P) + Carboplatin(C) with 66 Gray radiation) and arm B (two cycles of induction chemotherapy consisted of P+C followed by CCRT as of arm A. During treatment weekly, after completion of treatment at 6th week and thereafter 3 monthly evaluation was done till the end of study. S
tatistical analysis used: Chi-Square and Fisher Exact test did statistical analysis, t-test with 95%CI, Kaplan Meier survival analysis, Log Rank test using SPSS version 18.
Results: Among 44 patients, male (88.6%), Smokers (85.1%) were predominant with the most common histology was squamous cell carcinoma (52.4%). Overall response (Complete Response +Partial Response) was higher in Arm B 66.66% but statistically non-significant. Acute toxicities in both the arms were comparable and similar. DFS and PFS in the induction chemotherapy arm (Arm B) were numerically superior to concurrent chemoradiation arm (Arm A) but statistically nonsignificant
Conclusion: To conclude there were no significant differences in results between two arms in the present study population. Further studies with the larger sample size and longer duration of follow up are necessary.
Downloads
References
Magrath I, Litvak J. Cancer in developing countries: opportunity and challenge. JNCI: J Nat Cancer Inst. 1993;85(11):862-874. doi: https://doi.org/10.1093/jnci/85.11.862.
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA: Cancer J Clin. 2011;61(2):69-90. doi: https://doi.org/10.3322/caac.20107.
Loeb LA, Emster VL, Warner KE, Abbotts J, Laszlo J. Smoking and Lung Cancer: An Overview. Am Assoc Cancer Res.1984;44(12):5940-5958.
Zahm SH, Brownson RC, Chang JC, Davis JR. Study of lung cancer histologic types, occupation, and smoking in Missouri. Am J Indus Med. 1989;15(5):565-578. doi: https://doi.org/10.1002/ajim.4700150509.
Don L. Gibbons, Lauren A. Byers, and Jonathan M. Kurie. Smoking, p53 Mutation, and Lung Cancer.
Mol Cancer Res. 2014;12(1):3-13. doi: https://doi.org/10.1158/1541-7786.MCR-13-0539.
Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non–small-cell lung cancer to gefitinib. New Eng J Med. 2004;350(21):2129-2139. doi: https://doi.org/10.1056/NEJMoa040938.
Alberg AJ, Samet JM. Epidemiology of lung cancer. Chest. 2003;123(1):21S-49S. doi: https://doi.org/10.1378/chest.123.1_suppl.21s
Valaitis J, Warren S, Gamble D. Increasing incidence of adenocarcinoma of the lung. Cancer. 1981;47(5):1042-1046. doi: https://doi.org/10.1002/1097-0142(19810301)47:5%3C1042::aid-cncr2820470535%3E3.0.co;2-5.
Perez and Brady, Principles and Practice of Radiation Oncology,5th edition 2008.48:1083.
Schaake-Koning C, Van den Bogaert W. Dalesio 0, Festen J, Hoogenhout J, van Houtte P, Kirkpatrick A, Koolen M, Maat B, Nijs A. Effects of concomitant cisplatin and radiotherapy on inoperable non-small cell lung cancer. New Eng) J Med. 1992;326(8):524-530. doi: https://doi.org/10.1056/NEJM199202203260805.
Takeda K, Negoro S, Tanaka M, Fukuda H, Nakagawa K, Kawahara M, et al. A phase II study of cisplatin and irinotecan as induction chemotherapy followed by concomitant thoracic radiotherapy with weekly low-dose irinotecan in unresectable, stage III, non-small cell lung cancer: JCOG 9706. Japan J Clin Oncol. 2011;41(1):25-31. doi: https://doi.org/10.1093/jjco/hyq163.
Travis WD, Brambilla E, Nicholson AG, Yatabe Y, Austin JH, Beasley MB, et al. The 2015 World Health Organization classification of lung tumors: impact of genetic, clinical and radiologic advances since the 2004 classification. J Thorac Oncol. 2015;10(9):1243-1260. doi: https://doi.org/10.1097/JTO.0000000000000630.
Thun MJ, DeLancey JO, Center MM, Jemal A, Ward EM. The global burden of cancer: priorities for prevention. Carcinogenesis. 2010;31(1):100-110. doi: https://doi.org/10.1093/carcin/bgp263.
Sano H, Marugame T. International Comparisons of Cumulative Risk of Lung Cancer, From Cancer Incidence in Five Continents Vol. VIII. Japan J Clin Oncol. 2006;36(5):334-335. doi: https://doi.org/10.1093/jjco/hyl034.
Levin ML, Goldstein H, Gerhardt PR. Cancer and tobacco smoking: a preliminary report. J Am Med Assoc. 1950;143(4):336-338. doi: https://doi.org/10.1001/jama.1950.02910390008002.
Socinski MA, Rosenman JG, Halle J, Schell MJ, Lin Y, Russo S, et al. Dose‐escalating conformal thoracic radiation therapy with induction and concurrent carboplatin/paclitaxel in unresectable stage IIIA/B nonsmall cell lung carcinoma: a modified phase I/II trial. Cancer: Interdisciplinary Int J Am Cancer Soc. 2001;92(5):1213-1223. doi: https://doi.org/10.1002/1097-0142(20010901)92:5%3C1213::aid-cncr1440%3E3.0.co;2-0.
Vokes EE, Herndon JE, Crawford J, Leopold KA, Perry MC, Miller AA, et al. Randomized phase II study of cisplatin with gemcitabine or paclitaxel or vinorelbine as induction chemotherapy followed by concomitant chemoradiotherapy for stage IIIB non–small-cell lung cancer: Cancer and Leukemia Group B study 9431. J Clin Oncol. 2002;20(20):4191-4198. doi: https://doi.org/10.1200/JCO.2002.03.054.
Giorgio CG, Pappalardo A, Russo A, Santini D, Di Rosa C, Di Salvo C, et al. A phase II study of induction chemotherapy followed by concurrent chemoradiotherapy in elderly patients with locally advanced non-small-cell lung cancer. Anticancer drugs. 2007;18(6):713-719. doi: https://doi.org/10.1097/CAD.0b013e328082558a.
Lilenbaum R, Samuels M, Wang X, Kong FM, Jänne PA, Masters G, et al. A Phase II Study of Induction Chemotherapy Followed by Thoracic Radiotherapy and Erlotinib in Poor-Risk Stage III Non–Small-Cell Lung Cancer: Results of CALGB 30605 (Alliance)/RTOG 0972 (NRG). J Thorac Oncol. 2015;10(1):143-147. doi: https://doi.org/10.1097/JTO.0000000000000347.
Eng H. Tan, Joseph Wee, Peng T. Ang, Kam W. Fong, Swan S. Leong, Kei S. Khoo, et al. Induction Chemotherapy Followed by Concurrent Chemoradiotherapy in Stage III Unresectable Non-small Cell Lung Cancer. Acta Oncologica.2009;38(8):1005-1009. doi: https://doi.org/10.1080/028418699432266.
Pöttgen C, Eberhardt W, Bildat S, Stüben G, Stamatis G, Hillejan L, et al. Induction chemotherapy followed by concurrent chemotherapy and definitive high-dose radiotherapy for patients with locally advanced non-small-cell lung cancer (stages IIIa/IIIb): a pilot phase I/II trial. Ann Oncol. 2002;13(3):403-411. doi: https://doi.org/10.1093/annonc/mdf050
Kima MK, Kima SW, Choi EK, Sohn HJ, Lee DH, Suh C, et al. A1-01: Induction chemotherapy followed by concurrent chemoradiotherapy (CCRT) versus CCRT alone for unresectable stage III non-small cell lung cancer (NSCLC): randomized phase III trial. J Thorac Oncol.2007;2(8):S308. doi: https://doi.org/10.1097/01.JTO.0000283090.56099.af.
Belani CP, Choy H, Bonomi P, Scott C, Travis P, Haluschak J, et al. Combined chemoradiotherapy regimens of paclitaxel and carboplatin for locally advanced non-small-cell lung cancer: a randomized phase II locally advanced multi-modality protocol. J Clin Oncol. 2005;23(25):5883-5891. Epub 2005 Aug 8. doi: https://doi.org/10.1200/JCO.2005.55.405
Iranzo V, Bremnes RM, Almendros P, Gavila J, Blasco A, Sirera R et al. Induction chemotherapy followed by concurrent chemoradiation for patients with non-operable stage III non-small-cell lung cancer. Lung Cancer. 2009;63(1):63-67. doi: https://doi.org/10.1016/j.lungcan.2008.04.016.
Hirsh V, Soulieres D, Duclos M, Faria S, Vecchio PD, Ofiara L et al Phase II multicenter trial with carboplatin and gemcitabine induction chemotherapy followed by radiotherapy concomitantly with low-dose paclitaxel and gemcitabine for stage IIIA and IIIB non-small cell lung cancer. J Thorac Oncol. 2007;2(10):927-932. doi: https://doi.org/10.1097/JTO.0b013e3181560b92.


 OAI - Open Archives Initiative
OAI - Open Archives Initiative


