Assessment of efficacy of Anti-IgE (Omalizumab) therapy in patients with severe allergic asthma in a tertiary care hospital of Eastern India
Abstract
Introduction: Immunoglobulin E dependent mechanisms play an important role in the development of airway inflammation in allergic asthma. Atopic patients with severe asthma frequently have poorly controlled disease. Many have poor asthma control despite intensive treatment. Severe allergic asthma patients frequently treated with oral corticosteroids and therefore may develop serious side-effects. Anti-IgE antibody had been used in severe persistent allergic asthma in Western countries. However, its long-term efficacy in patients in India has not been reported.
Objective: To assess the efficacy of anti IgE therapy in patients with severe allergic asthma.
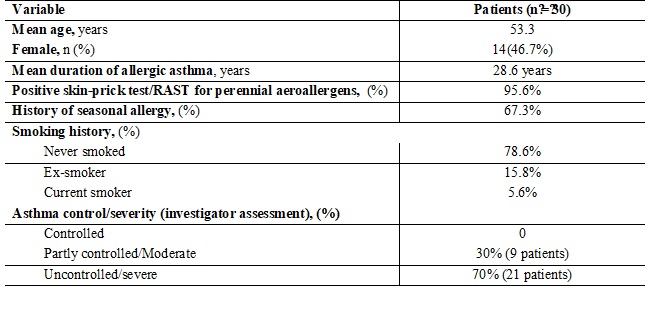
Method: 30 (16 male and 14 female) patients, with mean age of 49 having severe persistent allergic asthma, with recurrent exacerbations and on oral/IV steroids, received Omalizumab 150mg/300mg/450 mg for 1 year. Total dose of oral Steroids, use of rescue medications, changes in lung function (FEV1) were recorded at the baseline, 16 weeks & at end of the treatment (52 weeks) and then analyzed.
Results: Significant reduction observed in total oral steroid use at 16 week & at 52 weeks. -10.5mg (p<0.003) & 22.5mg respectively. Use of rescue medications decreased by -7.90 puffs(p- <0.001) at 16 weeks and by -13.67 puffs (13.67 (p -<0.001) at 52 weeks. Improvements in lung Function (FEV1) observed with a tune of 700 ml. from Baseline after 52 weeks therapy.
Conclusion: Use of anti-IgE antibody for 1 year is well tolerated and led to an overall significant improvement in patients with severe persistent allergic asthma.
Downloads
References
Humbert M, Beasley R, Ayres J, et al. Benefits of omalizumab as add-on therapy in patients with severe persistent asthma who are inadequately controlled despite best available therapy (GINA 2002 step 4 treatment): INNOVATE. Allergy. 2005 Mar;60(3):309-16. DOI: https://doi.org/10.1111/j.1398-9995.2004.00772.x.
Curtis JR, Westfall AO, Allison J, et al. Population-based assessment of adverse events associated with long-term glucocorticoid use. Arthritis Rheum. 2006 Jun 15;55(3):420-6. DOI: https://doi.org/10.1002/art.21984.
Hanania NA, Alpan O, Hamilos DL, et al. Omalizumab in severe allergic asthma inadequately controlled with standard therapy: a randomized trial. Ann Intern Med. 2011 May 3;154(9):573-82. doi: https://doi.org/10.7326/0003-4819-154-9-201105030-00002.
Normansell R, Walker S, Milan SJ, et al. Omalizumab for asthma in adults and children. Cochrane Database Syst Rev. 2014 Jan 13;(1):CD003559. doi: https://doi.org/10.1002/14651858.CD003559.pub4.
D'Amato G. Role of anti-IgE monoclonal antibody (omalizumab) in the treatment of bronchial asthma and allergic respiratory diseases. Eur J Pharmacol. 2006 Mar 8;533(1-3):302-7. Epub 2006 Feb 7.DOI: https://doi.org/10.1016/j.ejphar.2005.12.045.
Rodrigo GJ et al. Systematic review on the use of omalizumab for the treatment of asthmatic children and adolescents. Paediatric Allergy and Immunol 2015;26:551-6.
Molimard M, Buhl R, Niven R, et al. Omalizumab reduces oral corticosteroid use in patients with severe allergic asthma: real-life data. Respir Med. 2010 Sep;104(9):1381-5. doi: https://doi.org/10.1016/j.rmed.2010.06.001.
Walker S, Monteil M, Phelan K, Lasserson T, Walters E. Anti-IgE for chronic asthma in adults and children. Cochrane Database of Systematic Reviews. 2006;2:CD003559. doi: https://doi.org/10.1002/14651858.CD003559.pub3.
Rodrigo GJ, Neffen H, Castro-Rodriguez JA. Efficacy and safety of subcutaneous omalizumab vs placebo as add-on therapy to corticosteroids for children and adults with asthma: a systematic review. Chest. 2011 Jan;139 (1):28-35. doi: 10.1378/chest.10-1194. Epub 2010 Aug 5. DOI: https://doi.org/10.1378/chest.10-1194.
Lanier B, Bridges T, Kulus M, et al. Omalizumab for the treatment of exacerbations in children with inadequately controlled allergic (IgE-mediated) asthma. J Allergy Clin Immunol. 2009 Dec;124(6):1210-6. doi: https://doi.org/10.1016/j.jaci.2009.09.021.
Ohta K, Miyamoto T, Amagasaki T, et al. Efficacy and safety of omalizumab in an Asian population with moderate-to-severe persistent asthma. Respirology. 2009 Nov;14(8):1156-65. doi: https://doi.org/10.1111/j.1440-1843.2009.01633.x.
Hanania NA, Alpan O, Hamilos DL, et al. Omalizumab in severe allergic asthma inadequately controlled with standard therapy: a randomized trial. Ann Intern Med. 2011 May 3;154(9):573-82. doi: https://doi.org/10.7326/0003-4819-154-9-201105030-00002.
Bardelas J, Figliomeni M, Kianifard F, et al. A 26-week, randomized, double-blind, placebo-controlled, multicenter study to evaluate the effect of omalizumab on asthma control in patients with persistent allergic asthma. J Asthma. 2012 Mar;49(2):144-52. doi: https://doi.org/10.3109/02770903.2011.648296. Epub 2012 Jan 25.
Busse WW, Morgan WJ, Gergen PJ, et al. Randomized trial of omalizumab (anti-IgE) for asthma in inner-city children. N Engl J Med. 2011 Mar 17;364(11):1005-15. doi: https://doi.org/10.1056/NEJMoa1009705.
Molimard M, Le Gros V. Impact of patient-related factors on asthma control. J Asthma. 2008 Mar;45(2):109-13. doi: https://doi.org/10.1080/02770900701815727.
Korn S, Thielen A, Seyfried S, et al. Omalizumab in patients with severe persistent allergic asthma in a real-life setting in Germany. Respir Med. 2009 Nov;103(11):1725-31. doi: https://doi.org/10.1016/j.rmed.2009.05.002. Epub 2009 Jun 9.
Brusselle G, Michils A, Louis R, et al. "Real-life" effectiveness of omalizumab in patients with severe persistent allergic asthma: The PERSIST study. Respir Med. 2009 Nov;103(11):1633-42. doi: https://doi.org/10.1016/j.rmed.2009.06.014. Epub 2009 Jul 19.
Niven R, Chung KF, Panahloo Z, et al. Effectiveness of omalizumab in patients with inadequately controlled severe persistent allergic asthma: an open-label study. Respir Med. 2008 Oct;102(10):1371-8. doi: https://doi.org/10.1016/j.rmed.2008.06.002. Epub 2008 Jul 26.
Cazzola M, Camiciottoli G, Bonavia M, et al. Italian real-life experience of omalizumab. Respir Med. 2010 Oct;104(10):1410-6. doi: https://doi.org/10.1016/j.rmed.2010.04.013. Epub 2010 May 18.
Tzortzaki EG, Georgiou A, Kampas D, Lemessios M, Markatos M, Adamidi T, et al. Long-term omalizumab treatment in severe allergic asthma: The South- Eastern Mediterranean real-life experience. Pulm Pharmacol Therapeut. 2012;25(1):77–82.
Rottem M. Omalizumab reduces corticosteroid use in patients with severe allergic asthma: real-life experience in Israel. J Asthma. 2012 Feb;49(1):78-82. doi: https://doi.org/10.3109/02770903.2011.637598. Epub 2011 Dec 7.
Vennera Mdel C, Pérez De Llano L, Bardagí S, et al. Omalizumab therapy in severe asthma: experience from the Spanish registry--some new approaches. DOI: https://doi.org/10.3109/02770903.2012.668255.
Corren J, Casale TB, Lanier B, et al. Safety and tolerability of omalizumab. Clin Exp Allergy. 2009 Jun;39(6): 788-97. doi: https://doi.org/10.1111/j.1365-2222.2009.03214.x. Epub 2009 Mar 17.


 OAI - Open Archives Initiative
OAI - Open Archives Initiative


