A comparative study on efficacy and safety of glycopyrronium bromide + salmeterol/fluticasone and tiotropium bromide + salmeterol/fluticasone in chronic obstructive pulmonary disease
Abstract
Objective: To compare the effect of tiotropium bromide and glycopyrronium bromide in the treatment of chronic obstructive pulmonary disease.
Methods: This was an open labeled Randomized controlled trial study. Patients diagnosed with COPD according to the Global Initiative for chronic Obstructive Lung Disease (GOLD) strategy were included in the study. The patients were divided in two groups and each group had 100 patients. Group A- COPD patients on Tiotropium bromide + Salmeterol/Fluticasone; Group B – COPD patient on Glycopyrronium bromide + Salmeterol/Fluticasone. Tiotropium bromide: 18 mcg OD, Glycopyrronium bromide: 50 mcg OD along with Salmeterol 50 mcg/Fluticasone 100mcg was given.
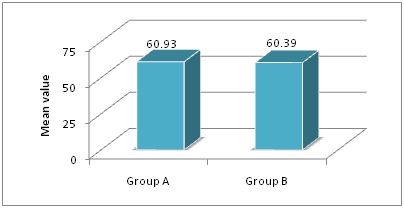
Results: The mean age of patients of Group A and Group B was 56.28±7.78 and 57.64±8.06 years respectively. Baseline variables were comparable between the groups. There was significant (p<0.05) difference in PFT parameters between the groups at 12 and 24 weeks except for FEV1/FVC. The mean change was higher in Group B compared to Group A from 0 week to 24 weeks. There was clinical improvement among all the patients in both the groups.
Conclusion: Once-daily GLY demonstrated similar effects to TIO when combined with SAL/FP in patients with moderate and severe COPD.
Downloads
References
Decramer M, Janssens W, Miravitlles M. Chronic obstructive pulmonary disease. Lancet. 2012 Apr 7;379(9823):1341-51. doi: https://doi.org/10.1016/S2213-2600(12)70060-7. Epub 2012 Feb 6.
Hogg JC, McDonough JE, Suzuki M. Small airway obstruction in COPD: new insights based on micro-CT imaging and MRI imaging. Chest. 2013 May;143(5):1436-1443. doi: https://doi.org/10.1378/chest.12-1766.
Pavkov R, Mueller S, Fiebich K, et al. Characteristics of a capsule based dry powder inhaler for the delivery of indacaterol. Curr Med Res Opin. 2010 Nov;26(11):2527-33. doi: https://doi.org/10.1185/03007995.2010.518916. Epub 2010 Sep 15.
Sykes DA, Dowling MR, Leighton-Davies J, et al. The Influence of receptor kinetics on the onset and duration of action and the therapeutic index of NVA237 and tiotropium. J Pharmacol Exp Ther. 2012 Nov;343(2):520-8. doi: https://doi.org/10.1124/jpet.112.194456. Epub 2012 Aug 1.
Bourbeau J, Christodoulopoulos P, Maltais F, et al. Effect of salmeterol/fluticasone propionate on airway inflammation in COPD: a randomised controlled trial. Thorax. 2007 Nov;62(11):938-43. Epub 2007 Jun 8.
Johnson M. Corticosteroids: potential beta2-agonist and anticholinergic interactions in chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2005;2(4):320-5; discussion 340-1.
Li Y, Li YH, Luo YW, et al. [Effects of inhaled short-acting bronchodilators on diaphragm function and neural respiratory drive during maximal isocapnic ventilation in patients with chronic obstructive pulmonary disease]. Nan Fang Yi Ke Da XueXue Bao. 2016 Feb;36(2):232-7.
Peter A Frith, Philip J Thompson, Rajeev Ratnavadivel, Catherina L Chang, Peter Bremner, Peter Day, Christina Frenzel, Nicol Kurstjens, the Glisten Study Group. Glycopyrronium once-daily significantly improves lung function and health status when combined with salmeterol/fluticasone in patients with COPD: the GLISTEN study—a randomized controlled trial. l. Thorax 2015; 70:519–527.
D'Urzo A, Bader G, Shen S, et al. Comparison of glycopyrronium versus tiotropium on the time to clinically important deteriorations in patients with COPD: a post-hoc analysis of randomized trials. NPJ Prim Care Respir Med. 2018 May 24;28(1):18. doi: https://doi.org/10.1038/s41533-018-0084-8.
The Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD), 2014.
Bourbeau J, Sebaldt RJ, Day A, et al. Practice patterns in the management of chronic obstructive pulmonary disease in primary practice: the CAGE study. Can Respir J. 2008 Jan-Feb;15(1):13-9.DOI: https://doi.org/10.1155/2008/173904.
White P, Thornton H, Pinnock H, et al. Overtreatment of COPD with inhaled corticosteroids--implications for safety and costs: cross-sectional observational study. PLoS One. 2013 Oct 23;8(10):e75221. doi: https://doi.org/10.1371/journal.pone.0075221. eCollection 2013.
Chapman KR, Beeh KM, Beier J, et al. A blinded evaluation of the efficacy and safety of glycopyrronium, a once-daily long-acting muscarinic antagonist, versus tiotropium, in patients with COPD: the GLOW5 study. BMC Pulm Med. 2014 Jan 17;14:4. doi: https://doi.org/10.1186/1471-2466-14-4.
Kerwin E, Hébert J, Gallagher N, et al. Efficacy and safety of NVA237 versus placebo and tiotropium in patients with COPD: the GLOW2 study. Eur Respir J. 2012 Nov;40(5):1106-14. doi: https://doi.org/10.1183/09031936.00040712. Epub 2012 Jul 26.
Beeh KM, Singh D, Di Scala L, et al. Once-daily NVA237 improves exercise tolerance from the first dose in patients with COPD: the GLOW3 trial. Int J Chron Obstruct Pulmon Dis. 2012;7:503-13. doi: https://dx.doi.org/10.2147%2FCOPD.S32451. Epub 2012 Jul 31.
Kew KM, Dias S, Cates CJ. Long-acting inhaled therapy (beta-agonists, anticholinergics and steroids) for COPD: a network meta-analysis. Cochrane Database Syst Rev. 2014 Mar 26;(3):CD010844. doi: https://doi.org/10.1002/14651858.CD010844.pub2.
Magnussen H, Watz H, Kirsten A, et al. Stepwise withdrawal of inhaled corticosteroids in COPD patients receiving dual bronchodilation: WISDOM study design and rationale. Respir Med. 2014 Apr;108(4):593-9. doi: https://doi.org/10.1016/j.rmed.2014.01.002. Epub 2014 Jan 15.
Xiang Min. Effect of tiotropium bromide combined with salmeterol fluticasone inhalation on airway function and airway inflammation in patients with moderate-severe stable COPD. Journal of Hainan Medical University 2016; 22(23): 24-27.
Wang T, Luo G, Hu Y, et al. Comparative study on the efficacy of tiotropium bromide inhalation and oral doxofylline treatment of moderate to severe stable chronic obstructive pulmonary disease. J Huazhong Univ Sci Technolog Med Sci. 2011 Oct;31(5):614. doi: https://doi.org/10.1007/s11596-011-0570-5. Epub 2011 Oct 25.
Oleksandr Varunkiv, Iryna Savelikhina. Tiotropium bromide: Efficacy and safety in complex treatment of COPD. European Respiratory Journal 2017; 50.


 OAI - Open Archives Initiative
OAI - Open Archives Initiative


