Antimicrobial Resistance and its Impact on Public Health
Mukherjee K1*
DOI:https://doi.org/10.17511/ijmrr.2024.i03.02
1* Kushal Mukherjee, MSc Microbiology, Department of Microbiology, Bidhannagar College, Kolkata, West Bengal, India.
Aim of the Study: The current study addresses the increasing threat of AMR and its direct impact on global public health. It aims to contribute to the existing knowledge about the key challenges of AMR, bringing attention to the need for further research and creating a combined effort in the battle with antimicrobial resistance.
Background and Methods: Antimicrobial resistance (AMR) has been considered one of the key problems that humankind has come across, showing a massive impact on public health globally. The continuous emergence of new microbial strains complicates it further by reducing the efficacy of the available antimicrobial drugs. For the current study, various scientific journals were studied from multiple resources. Furthermore, the websites of policymakers and agencies associated with this cause were studied and referred to.
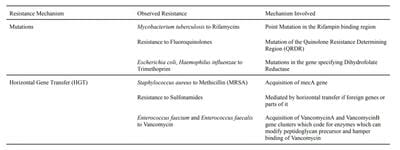
Results and Conclusions: The study has revealed a concerning trend of a steep increase in resistance of pathogens to antimicrobial agents. The major contributing factors which were identified during this study include misuse and overuse in the healthcare sector, inadequate prevention of infections and disease control, overuse in agriculture and the lack of novel antimicrobial agents. Several policies like the “One Health” approach by the Centers for Disease Control and Prevention (CDC) and the United Nations Sustainable Development Goals (SDGs) have been put in place as a means to combat the global public health problem. The study also highlights the need for policymakers, stakeholders and researchers to work in unison to combat the global issue.
Keywords: Antimicrobial Resistance, AMR, Healthcare, Public Health, Resistance
| Corresponding Author | How to Cite this Article | To Browse |
|---|---|---|
| , MSc Microbiology, Department of Microbiology, Bidhannagar College, Kolkata, West Bengal, India. Email: |
Mukherjee K, Antimicrobial Resistance and its Impact on Public Health. Int J Med Res Rev. 2024;12(3):72-83. Available From https://ijmrr.medresearch.in/index.php/ijmrr/article/view/1475 |


 ©
©