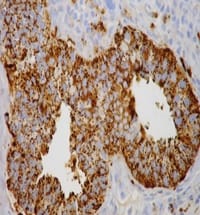
Role of liquid-based cytology and cell block study of pleural fluid in the evaluation of cases of malignant Pleural effusion with special reference to immunohistochemistry
Saha R.1, Sardar S.2, Das S.3*
DOI: https://doi.org/10.17511/ijmrr.2021.i03.09
1 Rama Saha, Associate Professor, IPGME & R and SSKM Hospital, Kolkata, West Bengal, India.
2 Samaresh Sardar, Medical Officer (Pathology), Mekhliganj S.D Hospital, Coochbehar, West Bengal, India.
3* Smritiparna Das, Post Graduate Trainee, Department of Pathology, IPGME & R and SSKM Hospital, Kolkata, West Bengal, India.
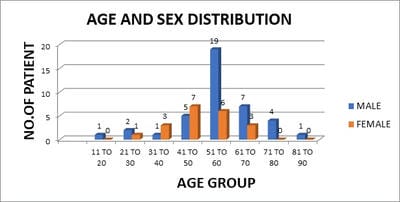
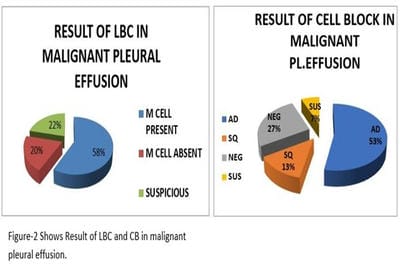
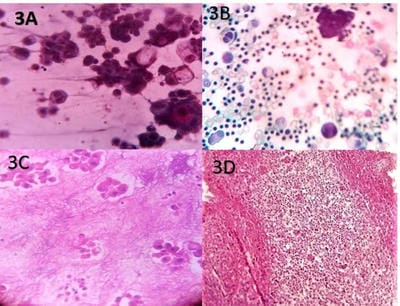
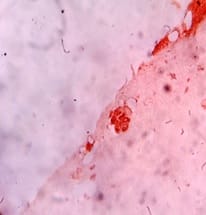
Introduction: Lung cancer is the most common primary tumor associated with malignant pleural effusion (MPE). In this study, we aim to use cell remnants for cell block preparation after performing liquid-based cytology (LBC) of effusion fluid. Immunohistochemistry was helpful to evaluate those cases having diagnostic dilemmas in LBC and cell block. Method: It was a cross-sectional, prospective, single institution-based study, conducted in the department of Pathology in collaboration with the Department of Respiratory Medicine IPGMER & SSKM Hospital, from January 2018 to June 2019 in the institution. Result: Most of the study population were in between the age group of 51 to 60 years with male predominance and with fever and cough being the predominant symptoms. Liquid-based cytology was positive for malignancy in 58% of cases and suspicious of malignancy in 22% of cases of malignant pleural effusion and it had 95.35% sensitivity, 58.82% specificity in diagnosing malignant pleural effusion.LBC was done followed by cell block preparations are studied further by Immunohistochemistry. Discussion: Morphological features were better identified by the cell block method when compared to LBC. Multiple sections can be obtained for special stain or IHC study which bridges the gap between cytology and histology.
Keywords: Cytological examination, Immunohistochemistry, Lung cancer, Malignant plural effusion
| Corresponding Author | How to Cite this Article | To Browse |
|---|---|---|
| , Post Graduate Trainee, Department of Pathology, IPGME & R and SSKM Hospital, Kolkata, West Bengal, India. Email: |
Saha R, Sardar S, Das S. Role of liquid-based cytology and cell block study of pleural fluid in the evaluation of cases of malignant Pleural effusion with special reference to immunohistochemistry. Int J Med Res Rev. 2021;9(3):185-192. Available From https://ijmrr.medresearch.in/index.php/ijmrr/article/view/1276 |


 ©
©