The sensitivity of electrodiagnostic criteria in subtype identification at the presentation in patients of Guillain-Barre syndrome
L. Mathukumalli N.1, Yaranagula S.2, A. Kanikannan M.3*, Chepuru R.4, Yareeda S.5, Sarva S.6, Borgohain R.7
DOI: https://doi.org/10.17511/ijmrr.2020.i02.01
1 Neeharika L. Mathukumalli, Assistant Professor, Department of Neurology, Nizam’s Institute of Medical Sciences, Hyderabad, Telangana, India.
2 Sai Deepak Yaranagula, Senior Resident, Department of Neurology, Nizam’s Institute of Medical Sciences, Hyderabad, Telangana, India.
3* Meena A. Kanikannan, Professor, Department of Neurology, Nizam’s Institute of Medical Sciences, Hyderabad, Telangana, India.
4 Ramesh Chepuru, Senior Resident, Department of Neurology, Nizam’s Institute of Medical Sciences, Hyderabad, Telangana, India.
5 Sireesha Yareeda, Assistant Professor, Department of Neurology, Nizam’s Institute of Medical Sciences, Hyderabad, Telangana, India.
6 Sailaja Sarva, Senior Lab Technologist, Department of Neurology, Nizam’s Institute of Medical Sciences, Hyderabad, Telangana, India.
7 Rupam Borgohain, Professor and Head of Department, DM Neurology, Department of Neurology, Nizam’s Institute of Medical Sciences, Hyderabad, Telangana, India.
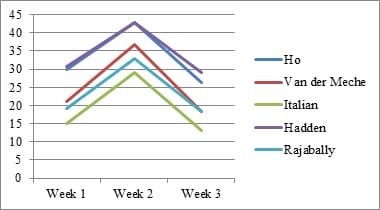
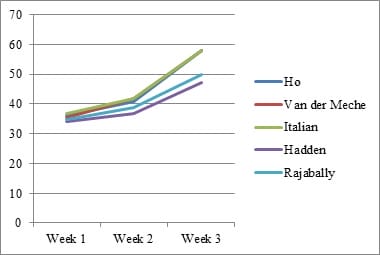
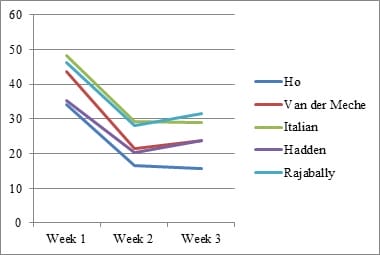
Introduction: Electrophysiology plays a pivotal role in identifying various GBS subtypes. Purpose: This study evaluates the sensitivity of 5 known electrophysiological criteria in patients with GBS at the time of presentation. Material & Methods: Clinical and electrophysiological data of GBS patients admitted with us between January 2011 and December 2016 were collected retrospectively from our hospital database, compiled and analyzed. Results: A total of 288 patients were included. Closer concordance was noted between the criteria in diagnosing axonal subtype (Range- 36.81% to 41.32%).Italian criteria had the highest sensitivity (41.32%). There was a wider variation in the diagnosis of AIDP (Range- 19.79 to 34.72%). Conclusion: As the timing of Nerve Conduction Studies (NCS) and the severity of disease influence the grouping of each patient into a specific electrophysiologic subtype, one should be cautious in interpreting electrodiagnosticdata.
Keywords: Electrophysiology in GBS, Sensitivity of electrophysiological criteria in GBS, Guillain Barre syndrome, GBS, Electrodiagnostic criteria
| Corresponding Author | How to Cite this Article | To Browse |
|---|---|---|
| , Professor, Department of Neurology, Nizam’s Institute of Medical Sciences, Hyderabad, Telangana, India. Email: |
Mathukumalli NL, Yaranagula SD, Kanikannan MA, Chepuru R, Yareeda S, Sarva S, Borgohain R. The sensitivity of electrodiagnostic criteria in subtype identification at the presentation in patients of Guillain-Barre syndrome. Int J Med Res Rev. 2020;8(2):140-147. Available From https://ijmrr.medresearch.in/index.php/ijmrr/article/view/1186 |


 ©
©