Assessment of levels of Vitamin D and Leptin in comparison of BMI among medical students
Sivakumar J.1*, Sampson U.2, Kumar J.3
DOI: https://doi.org/10.17511/ijmrr.2020.i01.08
1* Sivakumar J., Assistant Professor, Department of Biochemistry, Meenakshi Medical College, Kancheepuram, Tamil Nadu, India.
2 Ursula Sampson, Professor and Head, Department of Biochemistry, Meenakshi Medical College, Kancheepuram, Tamil Nadu, India.
3 Kumar J., Tutor, Department of Biochemistry, Meenakshi Medical College, Kancheepuram, Tamil Nadu, India.
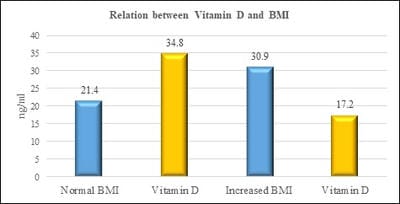
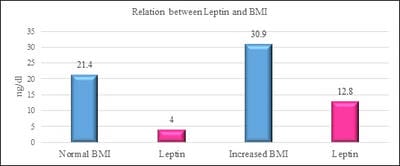
Introduction: Vitamin D is one of fat-soluble vitamin that plays an important role in the absorption of calcium and phosphate. Deficiency of Vitamin D is unrecognized in many parts of the world. Leptin is a hormone which is derived from adipose tissue. Studies have shown that vitamin D has a negative and powerful control on leptin secretion by vitamin D by acting on the adipose tissue. Aim and Objectives: The study was done to study the relationship between Vitamin D and Leptin based on Body mass index among the medical students. Materials and methods: Vitamin D Leptin and Body mass index were the parameters measured in the study group. Individuals with an age group of 19-23 years of both sexes were included in the study. Individuals above the age of 23 years, those with renal and liver disorders, individuals with hormonal disorders, individuals on vitamin D supplementation were excluded in the study. Vitamin D was measured by Enzyme-Linked Immunosorbent Assay (ELISA) method. Leptin was measured by Enzyme-Linked Immunosorbent Assay (ELISA) method. BMI is calculated by the formula weight in kilograms divided by height in metre square. Results: The results have shown that there is a decrease in vitamin D levels with increasing BMI. (pvalue≤0.001). furthermore, there is an increase in leptin levels with an increase in BMI. (pvalue≤0.001). Conclusion: The study has put forth a suggestion that leptin and vitamin D has a causal relationship between them based on Body Mass index. Adequate vitamin D levels will maximize the effect of maintaining normal leptin levels as high levels of leptin could contribute to obesity-related disorders.
Keywords: Vitamin D, Obesity, Leptin
| Corresponding Author | How to Cite this Article | To Browse |
|---|---|---|
| , Assistant Professor, Department of Biochemistry, Meenakshi Medical College, Kancheepuram, Tamil Nadu, India. Email: |
Sivakumar J, Sampson U, Kumar J. Assessment of levels of Vitamin D and Leptin in comparison of BMI among medical students. Int J Med Res Rev. 2020;8(1):51-56. Available From https://ijmrr.medresearch.in/index.php/ijmrr/article/view/1133 |


 ©
©