A prospective study comparing induction chemotherapy followed by chemoradiation versus chemoradiation alone in stage III non-small cell lung cancer
K Sardar P.1, R Shenoi L.2*
DOI: https://doi.org/10.17511/ijmrr.2020.i01.12
1 Pritam K Sardar, Clinical Tutor, Department of Radiotherapy, Malda Medical College and Hospital, Malda, West Bengal, India.
2* Lekshmi R Shenoi, Post Graduate Trainee, Department of Radiotherapy, IPGMER and SSKM Hospital, Kolkata, West Bengal, India.
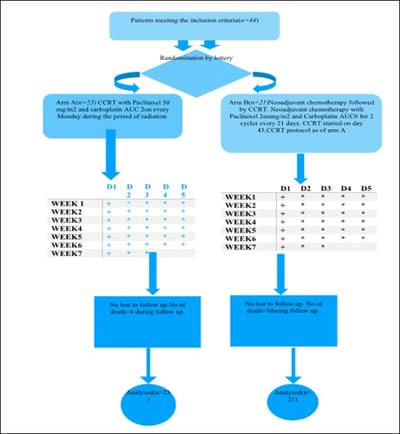
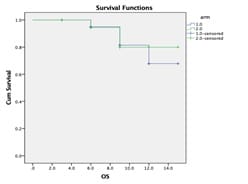
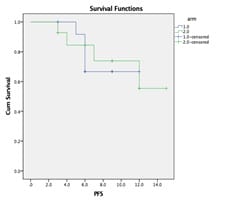
Background: Although concurrent chemoradiation (CCRT) is the standard of care for stage III non-small cell lung cancer(NSCLC), the five years overall (OS) survival is very poor. Most of the patients developed distant metastasis later which can be improved by induction chemotherapy. Aims: This study was designed to observe the difference in epidemiology, acute toxicities, overall responses [complete response (CR)+partial response (PR)] after treatment completion, disease-free survival (DFS) and progression-free survival (PFS) at the end of the study. Settings and Design: This was a prospective, interventional, randomized hospital-based study. Methods and Material: Eligible patients were randomized into arm A (CCRT with weekly paclitaxel(P) + Carboplatin(C) with 66 Gray radiation) and arm B (two cycles of induction chemotherapy consisted of P+C followed by CCRT as of arm A. During treatment weekly, after completion of treatment at 6th week and thereafter 3 monthly evaluation was done till the end of study. Statistical analysis used: Chi-Square and Fisher Exact test did statistical analysis, t-test with 95%CI, Kaplan Meier survival analysis, Log Rank test using SPSS version 18. Results: Among 44 patients, male (88.6%), Smokers (85.1%) were predominant with the most common histology was squamous cell carcinoma (52.4%). Overall response (Complete Response +Partial Response) was higher in Arm B 66.66% but statistically non-significant. Acute toxicities in both the arms were comparable and similar. DFS and PFS in the induction chemotherapy arm (Arm B) were numerically superior to concurrent chemoradiation arm (Arm A) but statistically nonsignificant Conclusion: To conclude there were no significant differences in results between two arms in the present study population. Further studies with the larger sample size and longer duration of follow up are necessary.
Keywords: Induction chemotherapy, Concurrent chemo-radiation, Lung cancer
| Corresponding Author | How to Cite this Article | To Browse |
|---|---|---|
| , Post Graduate Trainee, Department of Radiotherapy, IPGMER and SSKM Hospital, Kolkata, West Bengal, India. Email: |
Sardar PK, Shenoi LR. A prospective study comparing induction chemotherapy followed by chemoradiation versus chemoradiation alone in stage III non-small cell lung cancer. Int J Med Res Rev. 2020;8(1):76-85. Available From https://ijmrr.medresearch.in/index.php/ijmrr/article/view/1131 |


 ©
©