Classification, diagnosis of reversibility and severity assessment of patients having respiratory distress based on pulmonary function test
Ghosh S.1, Gayen P.2*
DOI: https://doi.org/10.17511/ijmrr.2020.i01.05
1 Saswata Ghosh, Associate Professor, Department of Chest Medicine, Malda Medical College, Malda, West Bengal, India.
2* Prosenjit Gayen, Associate Professor, Department of Pathology, Raiganj Government Medical College and Hospital, Raiganj, West Bengal, India.
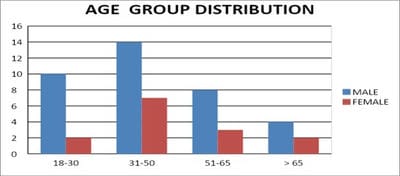
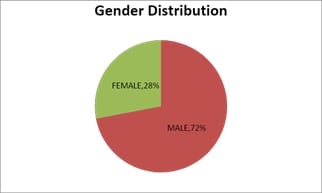
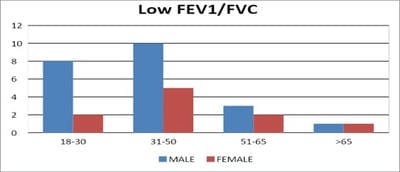
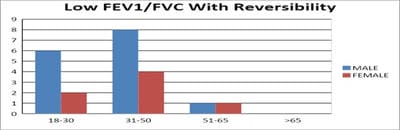
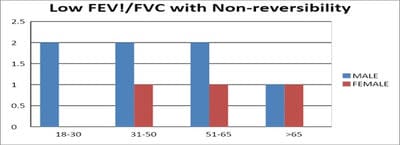
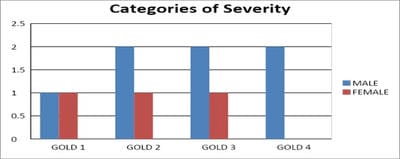
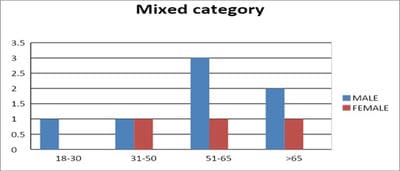
Introduction: Pulmonary function testing is the gold standard for physicians to diagnose and manage respiratory problems. An obstructive defect is indicated by low forced expiratory volume in one second/forced vital capacity (FEV1/FVC) ratio, defined as less than 0.7 or below the fifth percentile. If an obstructive defect is present, the physician should determine if the disease is reversible based on the increase in FEV1 or FVC after bronchodilator treatment (i.e., increase of more than 12% and 200 ml in adults). An FVC below the fifth percentile indicates a restrictive pattern based on NHANES III data in adults. If both the FEV1/FVC ratio and the FVC are low, the patient has a mixed defect. Method: A total of 60 patients having respiratory distress, who attended chest OPD underwent a pulmonary function test. Results: In this study out of 60 patients, 32 patients had obstructive airway diseases with low FEV1/FVC (53.33%), 8 of them (13.33%) had restrictive lung diseases, ten patients(16.66%) had mixed features and rest ten patients(16.66%) had normal spirometry. Among those 32 patients of obstructive features, 22 (68.75%) had reversible airway diseases. Severity was measured among the other ten non-reversible obstructive patients according to the GOLD criteria. Conclusion: Pulmonary function test is the fundamental first-line investigation to diagnose obstructive and restrictive lung diseases and also to differentiate between reversible and non-reversible obstruction. It is also a vital tool for determining the severity among non-reversible obstructive airway patients.
Keywords: Pulmonary function test, Obstructive airway disease, Restrictive lung disease
| Corresponding Author | How to Cite this Article | To Browse |
|---|---|---|
| , Associate Professor, Department of Pathology, Raiganj Government Medical College and Hospital, Raiganj, West Bengal, India. Email: |
Ghosh S, Gayen P. Classification, diagnosis of reversibility and severity assessment of patients having respiratory distress based on pulmonary function test. Int J Med Res Rev. 2020;8(1):31-39. Available From https://ijmrr.medresearch.in/index.php/ijmrr/article/view/1117 |


 ©
©