Analysis of central axis and build up region dose calculated by different dose calculation algorithms used for radiation treatment planning
Santhosh V.S.1*, Vishnu R.2
DOI: https://doi.org/10.17511/ijmrr.2019.i06.14
1* Santhosh V.S., Assistant Professor, Department of Radiation Physics, Government Medical College, Thiruvananthapuram, Kerala, India.
2 Vishnu R., Assistant Professor, Department of Radiation Physics, Government Medical College, Thiruvananthapuram, Kerala, India.
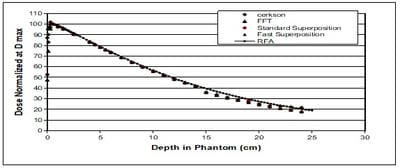
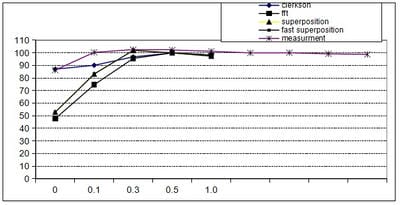
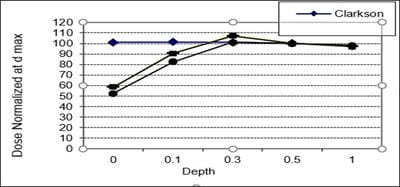
Introduction: Computerized Treatment planning system (TPS) is an integral part of radiation treatment procedure. The main part of treatment planning system is a calculation algorithm incorporated in the Treatment Planning System. Various algorithms used in the TPS have capabilities and limitation on specific situations. Oobjective: The objective of the work is to analyse the depth dose distribution generated by different dose calculation algorithms used in TPS in regions of low and high dose gradient. Materials and Methods: Four different dose calculation algorithms used for treatment planning such as Modified Clarkson, Sector integration, Fast Fourier Transform, Convolution- Superposition algorithm was used to generate the percentage depth dose distribution in a water phantom up to 25 cm depth. The values are compared with experimental results using Radiation Field Analyser. The PDD values also generated at build up region and the values were compared with experimental results using a Parallel plate ionization chamber. Rresults: The results show that the dose calculated by different algorithms shows difference at build up region and at larger depths. Conclusion: In the study, it was observed that the algorithms are reliable at the regions of dose uniformity, but care should be taken to interpret the dose distribution at the regions dose inhomogeneity.
Keywords: Radiation field analyser, Modified clarkson, Sector integration
| Corresponding Author | How to Cite this Article | To Browse |
|---|---|---|
| , Assistant Professor, Department of Radiation Physics, Government Medical College, Thiruvananthapuram, Kerala, India. Email: |
Santhosh VS, Vishnu R. Analysis of central axis and build up region dose calculated by different dose calculation algorithms used for radiation treatment planning. Int J Med Res Rev. 2019;7(6):536-542. Available From https://ijmrr.medresearch.in/index.php/ijmrr/article/view/1115 |


 ©
©