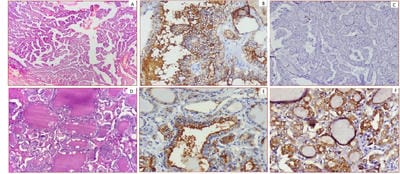
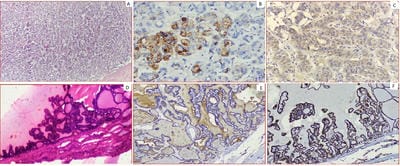
Application of Thyroid Peroxidase (TPO) and Hector Battifora Mesothelial-1 (HBME-1) immunohistochemical markers in the diagnosis of papillary thyroid carcinoma of the thyroid
Singh V.1, Kaur Bagga P.2*, Singh B.3, Jaideep4
DOI: https://doi.org/10.17511/ijmrr.2020.i01.17
1 Vasundhara Singh, Junior Resident, Department of Pathology, Government Medical College, Amritsar, Punjab, India.
2* Permeet Kaur Bagga, Associate Professor, Department of Pathology, Government Medical College, Amritsar, Punjab, India.
3 Bikramjeet Singh, Associate Professor, Department of Surgery, Government Medical College, Amritsar, Punjab, India.
4 Jaideep, Assistant Professor, Department of Pathology, Government Medical College, Amritsar, Punjab, India.
Background: Thyroid cancer is the most common endocrine malignancy accounting for >90% of malignancies of endocrine glands. The inter and intraobserver variation in the histomorphological diagnosis of Papillary Thyroid Carcinomas may sometimes pose a diagnostic difficulty. Application of IHC biomarkers may play an active or complementary role in their accurate classification. Aim: The present study was conducted to evaluate if HBME-1 and TPO immunohistochemical analysis can reliably differentiate papillary carcinomas from other thyroid lesions. Material and Methods: 50 cases of benign and malignant thyroid lesions were taken. Immunohistochemical staining for HBME-1 and TPO was performed. HBME-1 and TPO score was interpreted as absent and positive. Medical records were retrieved and their clinical data, surgical treatment, and pathological findings were noted. Results: Out of 50 cases, 19 (73.1%) cases were diagnosed PTC, 4 (15.4%) cases were FTC, 3(11.5%) cases were of MTC and 24 cases of benign thyroid lesions. TPO expression was found positive in 91.7% of cases of Benign thyroid lesions. In malignant thyroid lesions, negative expression of TPO was seen in 63.16%, 0% and 33.33% of PTC, FCT, and MCT respectively. Conclusion: Testing for expression of HBME-1 has been shown to improve the diagnostic accuracy for thyroid malignant nodules. The combination of HBME-1, and TPO may contribute to an accurate diagnosis of papillary thyroid carcinoma.
Keywords: TPO; HBME-1, Papillary Thyroid Carcinoma, Immunohistochemistry
| Corresponding Author | How to Cite this Article | To Browse |
|---|---|---|
| , Associate Professor, Department of Pathology, Government Medical College, Amritsar, Punjab, India. Email: |
Singh V, Bagga PK, Singh B, Jaideep. Application of Thyroid Peroxidase (TPO) and Hector Battifora Mesothelial-1 (HBME-1) immunohistochemical markers in the diagnosis of papillary thyroid carcinoma of the thyroid. Int J Med Res Rev. 2020;8(1):110-117. Available From https://ijmrr.medresearch.in/index.php/ijmrr/article/view/1111 |


 ©
©