A study of dexmedetomidine as an adjuvant to hyperbaric bupivacaine in subarachnoid block
Chauhan R.1, Ahmad S.2*, Bhattacharya S.3
DOI: https://doi.org/10.17511/ijmrr.2019.i06.11
1 Richa Chauhan, Senior Resident, Govind Ballabh Pant Institute of Postgraduate medical education and research, GIPMER, Delhi, India.
2* Sabih Ahmad, Senior Consultant, Department of Anaesthesiology, B.L. Kapoor Hospital, New Delhi, India.
3 S.K. Bhattacharya, Senior Consultant, Department of Anaesthesiology, Batra Hospital and Medical Research Centre, New Delhi, India.
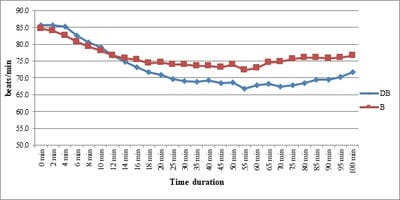
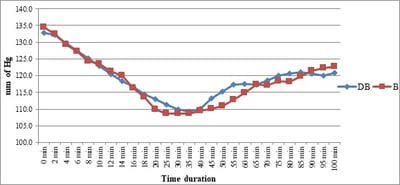
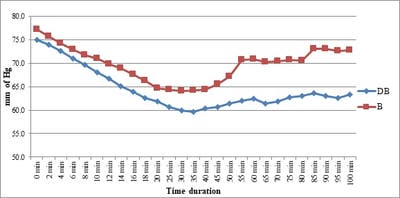
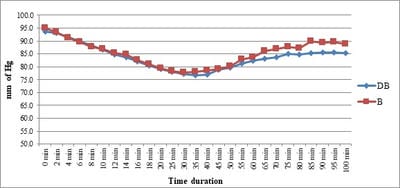
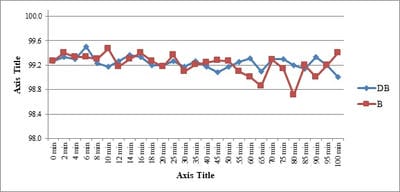
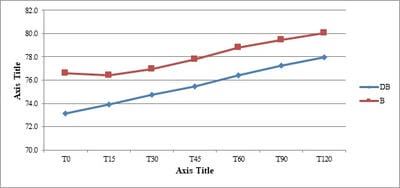
Background: No drug, used as adjuvant to spinal bupivacaine, has yet been identified that specifically inhibits nociception without its associated side-effects. Dexmedetomidine, a novel alpha-2 agonist which holds promise as an intra-thecal adjuvant. Aims: This observational study was conducted to evaluate the onset and duration of sensory and motor block as well as perioperative analgesia and adverse effects of dexmedetomidine given intrathecally with 0.5% hyperbaric bupivacaine for spinal anesthesia. Materials and Methods: A total of 60 patients belonging to age group 18-60 years, urban population, classified as American Society of Anesthesiologists status I and II scheduled for lower abdominal and lower limb procedures were prospectively studied. Patients were randomly allocated to receive intrathecally either 12.5 mg hyperbaric bupivacaine plus 5 μg (0.5 ml) dexmedetomidine (group D, n=30) or 12.5 mg hyperbaric bupivacaine plus 0.5 ml NS (group B, n=30). Sensory and motor blockade characteristics- The onset time to reach peak sensory and motor level, the regression time for sensory and motor block, time for rescue analgesia, hemodynamic changes and side-effects were recorded. Results: The onset times to reach T10 dermatome, peak sensory level and onset time to reach modified Bromage 3 motor block were similar in both groups. It was observed that adding dexmedetomidine intrathecally significantly prolonged sensory and motor block time. time for first analgesic request was also significantly prolonged in group BD. Statistically there were no significant differences in hemodynamic alterations and other adverse effects between the groups. Conclusion: clinical advantage of dexmedetomidine is that it facilitates the spread of the block and offers prolonged post‑operative analgesia. The groups were similar with respect to hemodynamic variables and there were no significant side-effects in either of the groups. However, prolonged duration of motor blockade with dexmedetomidine may be undesirable for short‑term surgical procedures or ambulatory surgeries.
Keywords: Dexmedetomidine, Bupivacaine, Spinal Anesthesia, Intrathecal, Adjuvant
| Corresponding Author | How to Cite this Article | To Browse |
|---|---|---|
| , Senior Consultant, Department of Anaesthesiology, B.L. Kapoor Hospital, New Delhi, India. Email: |
Chauhan R, Ahmad S, Bhattacharya SK. A study of dexmedetomidine as an adjuvant to hyperbaric bupivacaine in subarachnoid block. Int J Med Res Rev. 2019;7(6):511-519. Available From https://ijmrr.medresearch.in/index.php/ijmrr/article/view/1106 |


 ©
©