A single-centre experience of scrub typhus in a tertiary care centre - a re-emerging infection
Sahoo D.1*, Maikap D.2, Mohanty L.3, Patro S.4, Sekhar Panda S.5, Prasad Mohanty A.6
DOI: https://doi.org/10.17511/ijmrr.2020.i01.09
1* Debananda Sahoo, Assistant Professor, Department of Internal Medicine, All India Institute of Medical Sciences, Bhubaneswar, Odisha, India.
2 Debasish Maikap, Senior Resident, Department of Rheumatology, Kalinga Institute of Medical Sciences, Bhubaneswar, Odisha, India.
3 Lalatendu Mohanty, Professor, Department of Internal Medicine, Kalinga Institute of Medical Sciences, Bhubaneswar, Odisha, India.
4 Shubhransu Patro, Professor, Department of Internal Medicine, Kalinga Institute of Medical Sciences, Bhubaneswar, Odisha, India.
5 Sudhansu Sekhar Panda, Professor, Department of Internal Medicine, Kalinga Institute of Medical Sciences, Bhubaneswar, Odisha, India.
6 Ambika Prasad Mohanty, Professor, Department of Internal Medicine, Kalinga Institute of Medical Sciences, Bhubaneswar, Odisha, India.
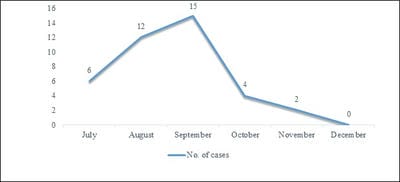
Background: Scrub typhus is a documented disease in Himachal Pradesh, but there have been few clinico-epidemiological studies in this area. The present study is done with IgM ELISA as a diagnostic test which has higher sensitivity and specificity as most of the previous studies had used Weil Felix test as a diagnostic test. Methodology: This was a prospective observational study. All the patients more than 18 years of age with positive IgM ELISA for scrub typhus with/without eschar were included. The clinical profile was observed. IgM scrub typhus was done by ELISA. Results: Total of 39 patients were observed between July 2016 to Dec 2016. Maximum patients were observed in August, September, and October. Fever with Headache was the most common presenting complaint. Eschar was present in 10 % patients. Complications were seen in 76.92 %. The mortality rate was 0 %. Conclusion: The varied presentations and high rate of complications require a high index of suspicion for Scrub Typhus. The general physicians should be sensitized for the early diagnosis and treatment to reduce morbidity and mortality.
Keywords: Scrub Typhus, Eschar, Orientia tsutsugamushi, Acute febrile illness
| Corresponding Author | How to Cite this Article | To Browse |
|---|---|---|
| , Assistant Professor, Department of Internal Medicine, All India Institute of Medical Sciences, Bhubaneswar, Odisha, India. Email: |
Sahoo D, Maikap D, Mohanty L, Patro S, Panda SS, Mohanty AP. A single-centre experience of scrub typhus in a tertiary care centre - a re-emerging infection. Int J Med Res Rev. 2020;8(1):57-62. Available From https://ijmrr.medresearch.in/index.php/ijmrr/article/view/1101 |


 ©
©