Case report- a case of Creutzfeldt-Jakob disease
Sontakke A.1, Khare B.2*, Srivastava B.3, Waran M.4, Tyagi B.5
DOI: https://doi.org/10.17511/ijmrr.2019.i04.01
1 Ajay Sontakke, Resident, Department of Medicine, DVVPF’s Medical College, Ahmednagar, Maharashtra, India.
2* Brig A. B. Khare (Retd), Associate Professor, Department of Medicine, DVVPF’s Medical College, Ahmednagar, Maharashtra, India.
3 Brig A.K. Srivastava (Retd), Associate Professor, Department of Medicine, DVVPF’s Medical College, Ahmednagar, Maharashtra, India.
4 Marcia Waran, Professor, Department of Medicine, DVVPF’s Medical College, Ahmednagar, Maharashtra, India.
5 Brig Arun Tyagi (Retd), Professor & HOD, Department of Medicine, DVVPF’s Medical College, Ahmednagar, Maharashtra, India.
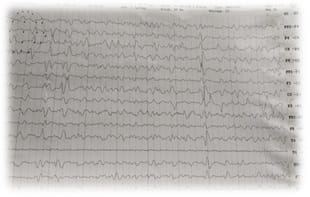
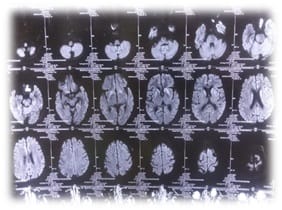
Creutzfeldt-Jakob Disease (CJD) is a rare invariably fatal neurodegenerative disease believed to be caused by an abnormal isoform of cellular infectious glycoprotein called prion protein. Though it is arare disease; yet it is the most common among prion diseases. Clinical presentation consists of rapidly progressive loss of memory, cognitive & visual disturbance, lack of coordination, myoclonus, cerebellar, pyramidal and extra pyramidal signs, akineticmutism & with progression of disease deterioration in higher mental functions become more pronounced. Periodic sharp triphasic wave complexes on EEG, high signal abnormalities in caudate nucleus and putamen on diffusion weighted (DW) or FLAIR MRI of Brain and positive 14-3-3 protein in CSF substantiate the diagnosis of CJD but definitive diagnosis is established by brain biopsy or autopsy materials. We report a case of 58-year old female patient who was admitted with complaints of rapidly progressive dementia, cognitive disturbance, blurring of vision and myoclonic jerks. Initial MRI brain and CSF findings were normal. Differential diagnoses that can present with rapidly progressive dementia and thereby mimic sporadic Creutzfeldt-Jakob disease were considered after review of literature. In EEG triphasic wave complexes were seen, repeat DWMRI after two weeks showed bilateral hyper-intensities in basal ganglia involving caudate nucleus and putamen, suggesting a diagnosis of probable CJD on the basis of center for disease control and prevention (CDC) criteria. The case is reported because of its rarity and also to emphasise that patients with rapidly progressive dementia, associated visual and cognitive disturbances and myoclonus should be investigated with DW MRI, EEG&CSF for diagnosis of CJD.
Keywords: Creutzfeldt-Jakob Disease (CJD), Progressive Dementia, Neurodegenerative, Prion Protein
| Corresponding Author | How to Cite this Article | To Browse |
|---|---|---|
| , Associate Professor, Department of Medicine, DVVPF’s Medical College, Ahmednagar, Maharashtra, India. Email: |
Sontakke A, Khare AB, Srivastava AK, Waran M, Tyagi A. Case report- a case of Creutzfeldt-Jakob disease. Int J Med Res Rev. 2019;7(4):253-258. Available From https://ijmrr.medresearch.in/index.php/ijmrr/article/view/1078 |


 ©
©