Evaluation of corticosteroid use in outpatient department of dermatology of a tertiary care teaching hospital: a prospective observational study
J. Sheth H.1, G. Chaudhary R.2*, D. Malhotra S.3
DOI: https://doi.org/10.17511/ijmrr.2019.i03.16
1 Haiya J. Sheth, Resident, Department of Pharmacology, Smt. N.H.L. Municipal Medical College, Ahmedabad, Gujarat, India.
2* Raju G. Chaudhary, Professor & Head, Department of Dermatology, Smt. N.H.L. Municipal Medical College, Ahmedabad, Gujarat, India.
3 Supriya D. Malhotra, Professor & Head, Department of Pharmacology, Smt. N.H.L. Municipal Medical College, Ahmedabad, Gujarat, India.
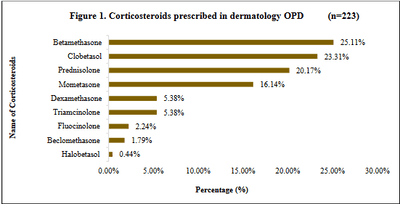
Background: Corticosteroids are widely prescribed drugs in dermatology. Rational prescribing of steroids is important for best therapeutic outcome at lowest possible dose. A study was carried out at a tertiary care teaching hospital in order to evaluate the use of corticosteroids which provided a picture of trends in the usage of corticosteroids in dermatology at that set-up. Materials and Methods: This prospective, observational study was carried out in department of dermatology for 1 year after ethical approval. Data was analysed for parameters related to corticosteroids, their potency, WHO drug prescribing indicators, effectiveness as well as effects of corticosteroids on quality of life of patients. Statistical analysis was done using Microsoft Excel Office 365. Results: In the 223 patients, 44.84% patients belonged to 21-40 years age group. Mostcommon indication was eczema in 29.15% cases. Topical betamethasone (25.11%) and oral prednisolone (20.17%) were most frequently prescribed. 95/140 topical steroids prescribed were super highly potent. Among concomitant drugs, a majority of 38% were antihistaminics. Degree of polypharmacy showed 04 drugs in a majority (43.15%) of prescriptions. Only 6.27% drugs were prescribed by generic name. Conclusion: Corticosteroids were beneficial to a large no. of patients. Initial usage of low potency steroids topically wherever possible can be emphasized. WHO drug prescribing indicators analysis indicated the need to adhere to WHO guidelines as well as prescribing drugs by generic name. To maintain a balance between judicious use and frequent abuse with corticosteroid is important along with physician’s vigilance and patient education.
Keywords: Betamethasone, Corticosteroids, Dermatology, Potency, Topical, WHO drug prescribing indicators
| Corresponding Author | How to Cite this Article | To Browse |
|---|---|---|
| , Professor & Head, Department of Dermatology, Smt. N.H.L. Municipal Medical College, Ahmedabad, Gujarat, India. Email: |
Sheth HJ, Chaudhary RG, Malhotra SD. Evaluation of corticosteroid use in outpatient department of dermatology of a tertiary care teaching hospital: a prospective observational study. Int J Med Res Rev. 2019;7(3):243-252. Available From https://ijmrr.medresearch.in/index.php/ijmrr/article/view/1066 |


 ©
©