Introduction
Chronic myelogenous leukaemia (CML) is myeloproliferative neoplasm consistently associated with BCR-ABL 1 fusion gene located in Philadelphia (Ph) chromosome [1]. The natural history is bi or triphasic(BP). The blastic phase is characterized by greater than 20% blasts in peripheral blood(PB), bone marrow,(BM) or extramedullary site[ 1]. The blast lineage is myeloid in 70% of cases. However basophilic blast crisis is extremely rare. We describe case of child,15yr old, with an initial presentation as basophilic blast crisis in CML. The other case was an adult 40year, known case of CML on follow-up, discontinued treatment due to financial constraints .and we discussed blastic phase of CML, along with these 2 rare basophilic blast crisis cases in detail laboratory features including clinical features, PS, BMA, FCI/IHC with note on BCR-ABLtranscripts review with available world literature.
Methods
We reviewed records of CML Between 2004 to 2012 received by Department of Pathology. Peripheral smear/BMA collected in suspected CML in blastic phase in 42 cases age ranged from youngest 9 years to oldest 67 years. , BMA /bone marrow biopsy of this blastic phase of CML was included in study. All non-blastic phases of CML were excluded. Bone marrow, Karyotyping and Molecular confirmation were available and IHC /FIC was also available in 15 cases. Bone marrow aspiration, cytogenetics, PCR and flow cytometry confirmed diagnosis. Clinical features were recorded. Anaemia was diagnosed when hemoglobin was < 10g dL. Leucocyte count >200X109/L.Thrombocytopenia< 150x109 . was seen in most cases, Reports of.IHC /FIC/BCR -ABL transcripts were reviewed.
Results
Abdominal fullness and pain were most common symptoms marked as splenomegaly, Adults and male preponderance were seen. Splenomegaly was predominant finding occurring in 95% of patients. The median size measured on ultrasound was 17 cm. Mild to moderate hepatomegaly on USG was seen in most patients. Blood tests for HIV, HBsAg and HCV were negative. Serum LDH levels range normal 35U/L to as high as 77800/L.Serum Alkaline phosphatase was within normal range in all cases. Hemoglobin value ranged from (5.5-10.g/dL). Anaemia, WBC count raised, thrombocytopenia.was frequently seen. CML was Classified according to WHO criteria, Bone marrow analysis revealed a blastic phase (fig1) Bone marrow basophilia 6% was seen in all blast crisis cases.
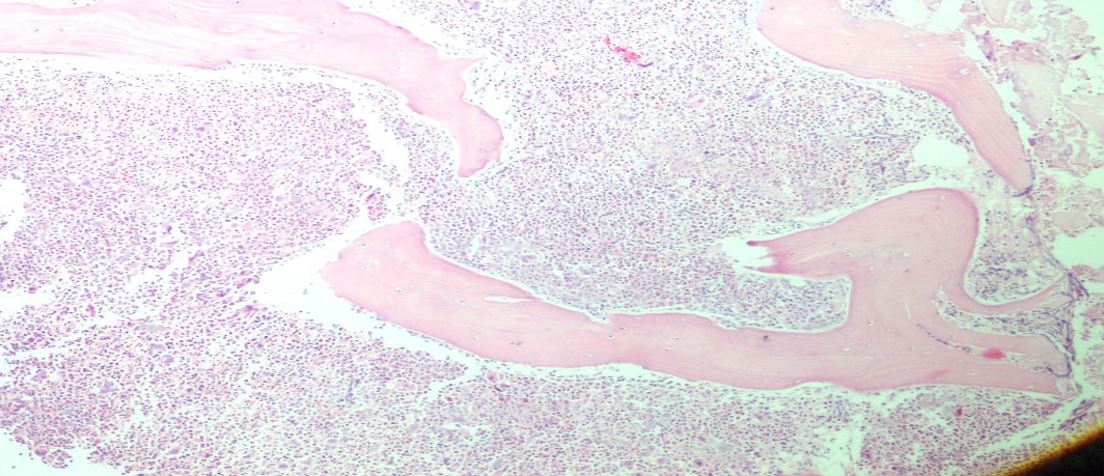
Figure 1: 20x H&E Showing increased blasts in bone marrow
Blast varied from 20% to 70% in most cases, myeloid BP showed myeloid markers positivity and lymphoid BP revealed lymphoid markers positivity. 2 cases had basophilic blast crisis, basophil 40 to 60% along with blast of 30%. First case of basophilic blast crisis was 15-year-old boy who was referred to our institute,
tertiary cancer centre in South India with history of fatigue and abdominal mass. On examination of salient clinical findings of pallor, icterus and massive splenomegaly, no symptoms attributable to increased histamine such as urticaria, peptic ulcer or asthma were reported. Hemogram hemoglobin 7.4g/dl, total count,2.34,300/cumm, with differential count of >blast 30 %, basophil 60%, platelet count 1,14,000 /cumm. LFT was within normal range except for total bilirubin 3mg/dl. Serum electrolytes, renal function tests, blood glucose levels, LDH, and Uric acid were within normal limits. Chest X-ray was normal. Toludine blue showed metachromatic granules characteristic of basophil. BM examination was done which showed higher blast count of 65 %.Cytogenetic analysis revealed karyotype of 47XY,t(9;22)(q34:q11.2),+der(9)t(9;22)(q34;11.2),i(17)(q10)(06)/46,XY,t(9;22)(q34;q11.2)(04).BM was subjected to flow cytometry, Followed by IHC on bone marrow biopsy blasts expressed CD 34, myeloid markers, CD117, CD38, and MPO (fig 2) and were negative for Tdt, CD5, CD3, CD7, CD10, CD79b, CD20 (fig3)

Figure 2: 20x MPO positive blasts
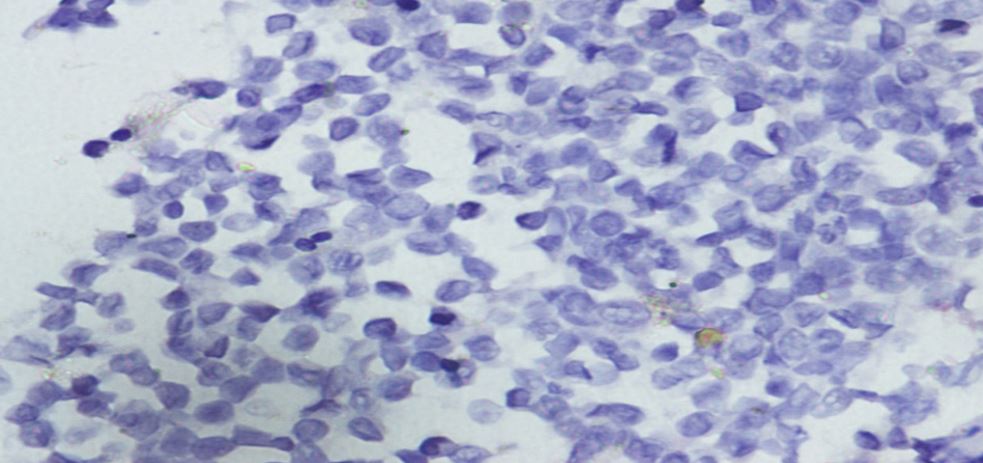
Figure 3: CD20 being negative.
PCR analysis showed BCR/ABL P210 and transcript, diagnosis of basophilic blast crisis was made and was started on chemotherapy and Imatinib 400mg/day. After > year of follow up patient is well and in clinical, hematological and molecular remission. Another case was 40-year-old man who presented with fatigue and a mass abdomen. On examination of salient clinical findings of pallor, and massive splenomegaly, no symptoms attributable to increased histamine such as urticarial, peptic ulcer or asthma were reported. Hemogram hemoglobin 9.4g/dl, total count,3.39,300/cumm, with differential count of blast 40 %, basophil 40%, platelet count 1,14,000 /cumm. LFT was within normal range. Serum electrolytes, renal function tests, blood glucose levels, LDH, and Uric acid were within normal limits. Chest X-ray was normal. Toludine blue showed metachromatic granules characteristic of basophil. BM examination was done which showed blast count of 40 % (, fig 4)karyotyping was not done, and patient was lost for follow-up.
Discussion
Most cases of CML terminate in blast crisis typically as myeloid blast crisis.1/3rd are lymphoblastic, less commonly seen are erythroblasts, monocytic, and barely encountered are basophilic blast crises.[ 3&4]


 ©
©