A study of her-2/ neu oncogene expression in benign and malignant ovarian tumors
Gupta S.1*, Mehra M.2, Khattri J.3, M.4
DOI: https://doi.org/10.17511/ijmrr.2020.i02.06
1* Sumit Gupta, Associate Professor, Department of Pathology, N.I.M.S Medical College, Jaipur, Rajasthan, India.
2 Manju Mehra, Professor, Department of Pathology, N.I.M.S Medical College, Jaipur, Rajasthan, India.
3 Jyotsana Khattri, Assistant Professor, Department of Pathology, N.I.M.S Medical College, Jaipur, Rajasthan, India.
4 Madhvi , Junior Resident, Department of Pathology, N.I.M.S Medical College, Jaipur, Rajasthan, India.
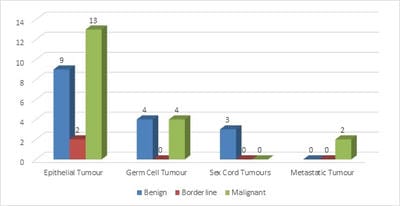
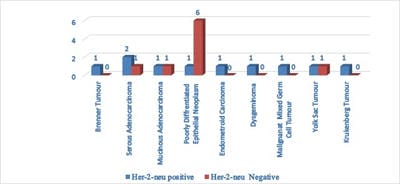
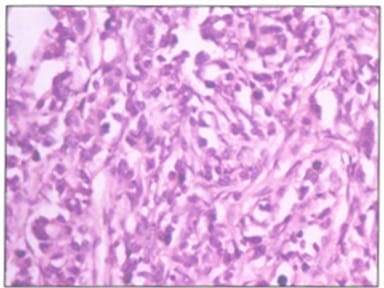
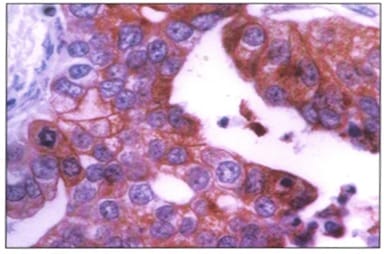
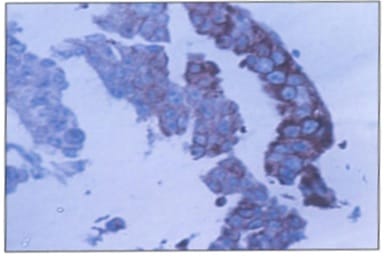
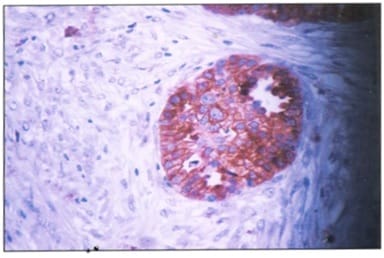
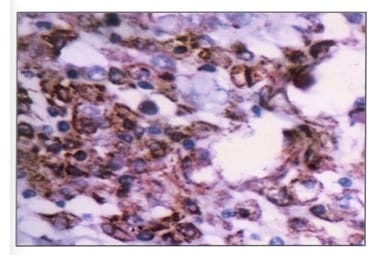
Background: Ovarian cancers are very common worldwide with serous epithelial tumors being the most common. Her-2/neuprotoncogene encodes a protein belonging to the EGFR tyrosine kinase receptor family. Overexpression has been shown for poor prognosis in breast cancer. The study was done to find the association of ovarian tumors with Her-2/neu expression. Aim and objectives: To assess the clinicopathological profile of various ovarian tumors with special reference to age, histological type, grade, and stage of the tumor. To assess and compare the expression of Her-2/neu oncogene in benign and malignant ovarian tumors in relation to age, histological type, grade, and stage of the tumor. Method: The prospective study was done on 37 specimens received in the Department of Pathology; NIMS medical college from the period between 2015 to 2019. Results: All the benign and borderline tumors were negative for her-2/neu .48.6% of malignant tumors were her-2/neu positive. Conclusion: Her-2/neu positivity was seen in 24.3% of ovarian tumors. All the benign and borderline tumors were negative for her-2/neu. 48.6% of malignant tumors were her-2/neu positive.
Keywords: Her-2/neuprotoncogene, Ovarian cancer, Epithelial tumors
| Corresponding Author | How to Cite this Article | To Browse |
|---|---|---|
| , Associate Professor, Department of Pathology, N.I.M.S Medical College, Jaipur, Rajasthan, India. Email: |
Gupta S, Mehra M, Khattri J, Madhvi M. A study of her-2/ neu oncogene expression in benign and malignant ovarian tumors. Int J Med Res Rev. 2020;8(2):169-175. Available From https://ijmrr.medresearch.in/index.php/ijmrr/article/view/1181 |


 ©
©