Dosimetric evaluation of 6 MV and 18 MV intensity-modulated radiotherapy plans for treatment of carcinoma of the cervix
Palaniappan SM.1, Khaleel I.2*, Varatharaj C.3, Ganesh K M4, Ravikumar M.5
DOI: https://doi.org/10.17511/ijmrr.2020.i04.04
1 Senthil Manikandan Palaniappan, Assistant Professor, Department of Radiation Physics, Kidwai Memorial Institute of Oncology, Bangalore, Karnataka, India.
2* Ibrahim Khaleel, Assistant Professor, Department of Radiation Physics, Kidwai Memorial Institute of Oncology, Bangalore, Karnataka, India.
3 Varatharaj C., Associate Professor, Department of Radiation Physics, Kidwai Memorial Institute of Oncology, Bangalore, Karnataka, India.
4 Ganesh K M, Professor, Department of Radiation Physics, Kidwai Memorial Institute of Oncology, Bangalore, Karnataka, India.
5 Ravikumar M., Professor, Department of Radiotherapy, Sri Shankara Cancer Foundation, Bangalore, Karnataka, India.
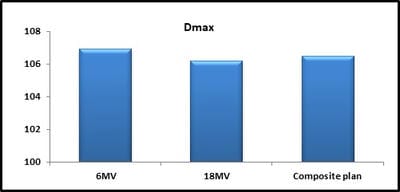
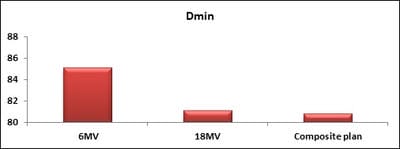
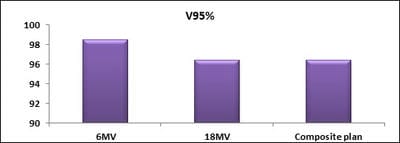
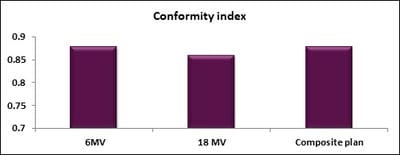
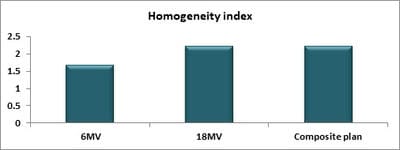
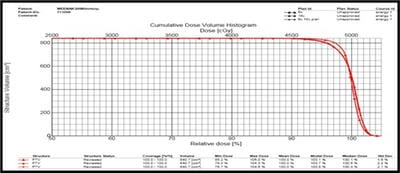
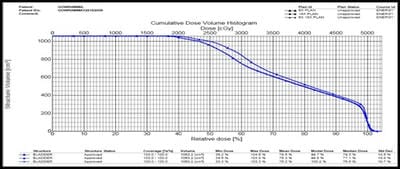
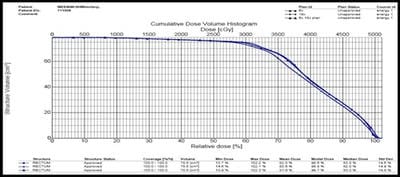
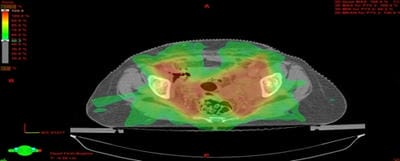
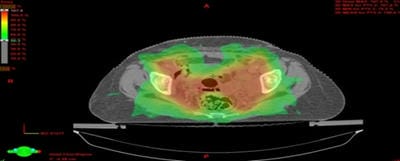
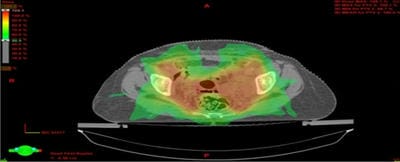
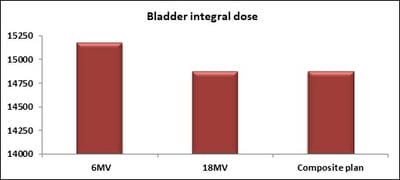
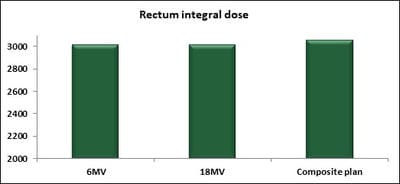
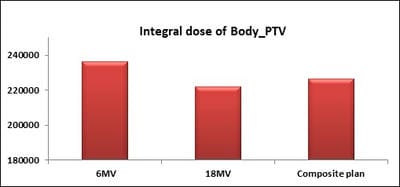
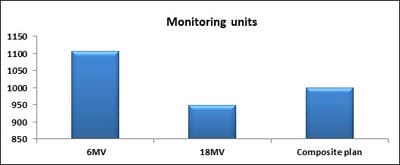
Introduction: Cervical cancer (Ca Cx) is the fourth most frequent cancer in women with an estimated 57000 new cases in 2018 representing 6.6% of all female cancers. Approximately 90% of deaths from cervical cancer occurred in low- and middle-income countries. Material and Methods: A retrospective radiotherapy treatment planning comparative study conducted at the Department of Radiation Physics, Kidwai Memorial Institute of Oncology, Bangalore during June 2018- March 2019. Result: All the plans were normalized to 100 % at Target mean to achieve a similar target dose for quantitative comparison of DVHs. The results for target coverage, OAR sparing, integral dose, and monitoring units. Conclusions: The tradeoff of using 6 MV and 18 MV for cervix patients depends on many parameters. Since the same PTV coverage was forced for both energies by having the same optimization constraints, there was little difference in target coverage and conformity index for both energies.
Keywords: Cervical cancer, Intensity-modulated radiation therapy, Dosimetric plan
| Corresponding Author | How to Cite this Article | To Browse |
|---|---|---|
| , Assistant Professor, Department of Radiation Physics, Kidwai Memorial Institute of Oncology, Bangalore, Karnataka, India. Email: |
Palaniappan SM, Khaleel I, Varatharaj C, Ganesh K M, Ravikumar M. Dosimetric evaluation of 6 MV and 18 MV intensity-modulated radiotherapy plans for treatment of carcinoma of the cervix. Int J Med Res Rev. 2020;8(4):307-314. Available From https://ijmrr.medresearch.in/index.php/ijmrr/article/view/1142 |


 ©
©