Helicobacter pylori chronic gastritis: correlation between endoscopic findings and histopathology with special reference to updated sydney system
Banik T.1, Kumar Bar P.2*, Mandal S.3
DOI: https://doi.org/10.17511/ijmrr.2020.i01.02
1 Tarak Banik, Assistant Professor, Malda Medical College, Malda, West Bengal, India.
2* Prasenjit Kumar Bar, Assistant Professor, Malda Medical College, Malda, West Bengal, India.
3 Saikat Mandal, Demonstrator, Malda Medical College, Malda, West Bengal, India.
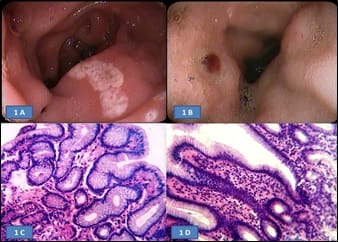
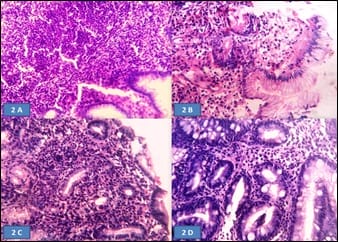
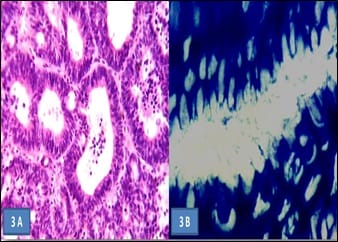
Aims of the study: Helicobacter pylori (H. pylori) induces inflammatory changes in the gastric mucosa variably correlated to different endoscopic and histologic features. The present prospective study aimed to correlate different endoscopic findings with histomorphological changes and the presence of H. pylori in the gastro-duodenal mucosa, in samples of dyspeptic patients. Methods: 60 dyspeptic patients of 18 years to 60 years of age were selected from outpatient department and screened with gastro-duodenoscopy and biopsy. The presence of H. pylori was determined by urease test on fresh biopsy specimens and histologically using the modified Giemsa stain. Findings were recorded and analyzed statistically. Results: Highest (84.6%) H. pylori positivity was seen in the 41-50 years age group. Majority of the patients had a normal upper gastro-intestinal endoscopy; among them majority (61.2%) was positive for H. pylori infection. Most cases with endoscopic lesion in the gastro-duodenal mucosa were also positive for H. pylori infection. On biopsy, chronic gastritis was the most common (73.33%) finding in 44 cases, among them, more than two-third (70.4%) were positive for H. pylori. Conclusion: H. pylori gastritis is strongly associated with peptic ulcer diseases, chronic gastritis and non-ulcer dyspepsia. Endoscopy and biopsy play the main role in diagnosis and identification of the spectrum of involvement.
Keywords: H. Pylori, Endoscopy, Histopathology
| Corresponding Author | How to Cite this Article | To Browse |
|---|---|---|
| , Assistant Professor, Malda Medical College, Malda, West Bengal, India. Email: |
Banik T, Bar PK, Mandal S. Helicobacter pylori chronic gastritis: correlation between endoscopic findings and histopathology with special reference to updated sydney system. Int J Med Res Rev. 2020;8(1):7-13. Available From https://ijmrr.medresearch.in/index.php/ijmrr/article/view/1132 |


 ©
©