Clinical Profile of MDR-TB patients with special reference to kanamycin induced ototoxicity in a tertiary care hospital of eastern India
Ghosh S.1, Gayen P.2*, Halder A.3, Bandyopadhyay R.4
DOI: https://doi.org/10.17511/ijmrr.2019.i03.12
1 Saswata Ghosh, Assistant Professor, Department of Chest Medicine, Malda Medical College and Hospital, Malda, West Bengal, India.
2* Prosenjit Gayen, Assistant Professor, Department of Pathology, Malda Medical College and Hospital, Malda, West Bengal, India.
3 Atish Halder, Assistant Professor, Department of ENT, Malda Medical College and Hospital, Malda, West Bengal, India.
4 Ramtanu Bandyopadhyay, Professor, Department of General Medicine, Malda Medical College and Hospital, Malda, West Bengal, India.
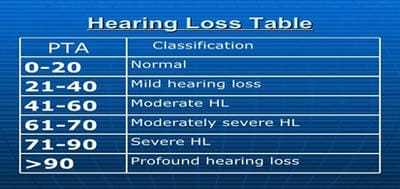
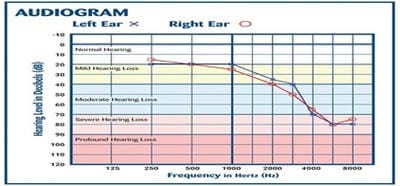
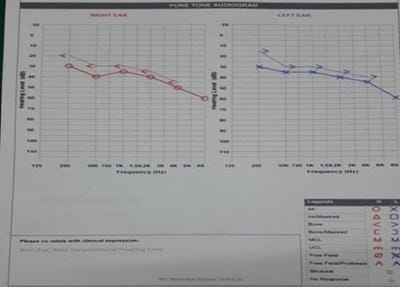
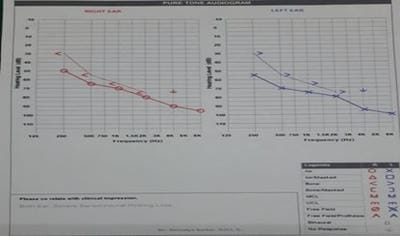
Introduction: MDR-TB is defined as resistance to isoniazid and rifampicin, with or without resistance to other anti-TB drugs. Multidrug-resistant TB (MDR-TB) remains a public health crisis and a health security threat. Kanamycin, is an aminoglycoside antibiotic used to treat multi-drug resistant TB in the intensive phase. Objective: To analyze the patients of MDR-TB with respect to age, sex and presence of comorbidities like diabetes mellitus. Also to study the incidence of hearing impairments among patients of MDR-TB receiving injectable Kanamycin. Methods: 40 patients of MDR-TB diagnosed by sputum culture and drug susceptibility testing (DST) have been classified on the basis of age, sex and presence of diabetes mellitus. All have received injectable Kanamycin for 6months in their intensive phase (IP). Patients giving history of auditory impairments underwent pure tone audiometry (PTA) for detection of sensory neural hearing loss, if any. Result: Out of 40 patients of MDR-TB, 30 were males and the rest 10 were females. Age ranges from 12 to 70 years among which maximum patients fell in the age group of 21-30 years (12 patients). 16 patients were diabetic. After getting Kanamycin, 8 patients gave the history of auditory disturbances and only 1 patient found to have severe sensory neural hearing loss confirmed by pure tone audiometry.Conclusion: Prevalence of MDR-TB has been found more among males and in younger age group. Diabetes Mellitus play a major role here. Kanamycin induced hearing loss is not a very serious concern in our study.
Keywords: Deafness, Kanamycin, Pure tone audiometry, Multidrug-resistant tuberculosis
| Corresponding Author | How to Cite this Article | To Browse |
|---|---|---|
| , Assistant Professor, Department of Pathology, Malda Medical College and Hospital, Malda, West Bengal, India. Email: |
Ghosh S, Gayen P, Halder A, Bandyopadhyay R. Clinical Profile of MDR-TB patients with special reference to kanamycin induced ototoxicity in a tertiary care hospital of eastern India. Int J Med Res Rev. 2019;7(3):218-223. Available From https://ijmrr.medresearch.in/index.php/ijmrr/article/view/1062 |


 ©
©