Open label case control prospective study to evaluate the impact of aceclofenac on antihypertensive action of amlodipine and ramipril in hypertensive patients with osteoarthritis
Babu Raman R.1, Khare A.2*, Kumar D.3, Ghosh D.4, Tripathi N.5, Srivastava D.6, Prakash V.7, Saurabh S.8
DOI: https://doi.org/10.17511/ijmrr.2019.i01.08
1 Ram Babu Raman, Tutor, 2* A.K. Khare, Associate Professor, 3 Deepak Kumar, Tutor, 4 Dibyadeb Ghosh, The Head, 5 Neeraj Tripathi, Assistant Professor, Department of Medicine, 6 Deepak Srivastava, Assistant Professor, Department of Orthopaedics Surgery, Hind Institute of Medical Sciences, Barabanki, Uttar Pradesh, India.
7 Ved Prakash, Assistant Professor, 8 Seemant Saurabh, Assistant Professor; All the authors are affiliated with the Department of Pharmacology, Hind Institute of Medical Sciences, Barabanki, Uttar Pradesh, India.
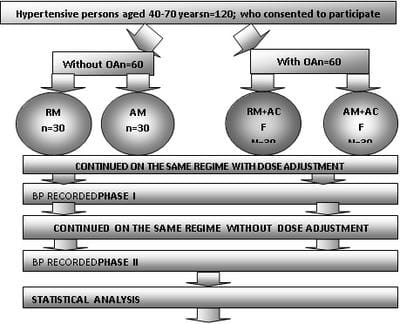
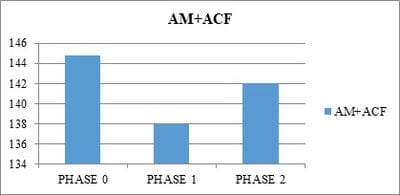
Objective: To study the impact of aceclofenac on antihypertensive action of amlodipine and ramiprilin hypertensive patients with osteoarthritis in an open label case control prospective study. Methods: This was an open label case control prospective study. Hypertensive patients on either amlodipine or Ramipril were included in control groups. A total of 120 patients were included in the study and divided into 4 groups: Group A- Hypertensivepatients on Ramipril; Group B- Hypertensive patients with concomitant osteoarthritis taking Aceclofenac and Ramipril; Group C– Hypertensive patients on Amlodipine and Group D- Hypertensive patients with concomitant osteoarthritis taking Aceclofenac and Amlodipine. Results: At the end of the first month (phase I) the ramipril subgroup in the control group had a mean systolic blood pressure of 136.73±3.80 which was an 8.19% decrease from the baseline and it was found significant (p<0.05). The systolic blood pressure measurements at the end of the second month (phase II) in the control groups revealed further fall in mean systolic blood pressure. The cases of osteoarthritis on aceclofenacand ramiprilshowedan increase in BP. The mean Systolic BP was 159.2 ± 5.816. An increase of 9.74% from the base line and 16.09% was noted at the end of phase-I (P<0.5). Conclusion: The interaction of NSAIDs on the antihypertensive action of the ACE inhibitors is significantly greater than their blunting action on the calcium channel blockers.
Keywords: Hypertension, Antihypertensive drugs, ACE inhibitors
| Corresponding Author | How to Cite this Article | To Browse |
|---|---|---|
| , Associate Professor, Department of Pharmacology, Hind Institute of Medical Sciences, Barabanki, Uttar Pradesh, India. Email: |
Raman R, Khare AK, Kumar D, Ghosh D, Tripathi N, Srivastava D, Prakash V, Saurabh S. Open label case control prospective study to evaluate the impact of aceclofenac on antihypertensive action of amlodipine and ramipril in hypertensive patients with osteoarthritis. Int J Med Res Rev. 2019;7(1):43-49. Available From https://ijmrr.medresearch.in/index.php/ijmrr/article/view/1034 |


 ©
©